Содержание
- 2. Gene Delivery Ex vivo Transplantation of recombinant cells with virus Direct administration of virus In vivo
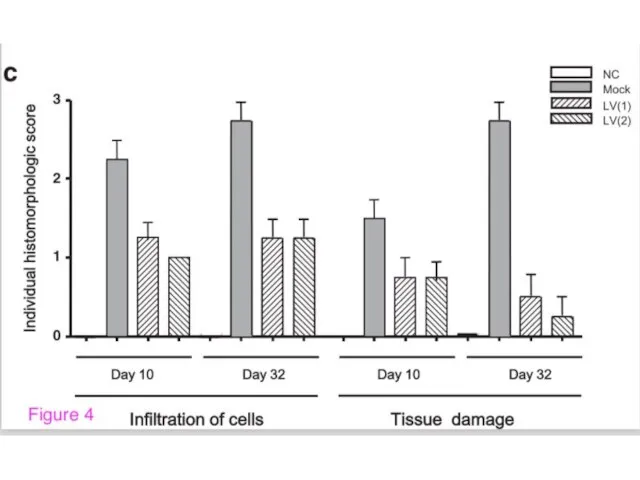
- 3. “New” Gene Therapy Mucosal gene therapy using a pseudotyped lentivirus vector encoding murine interleukin-10 (mIL-10) suppresses
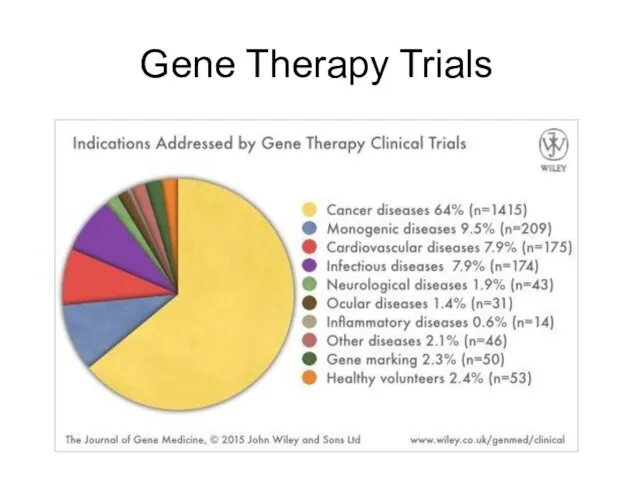
- 5. Gene Therapy Trials
- 6. Gene Therapy Ex vivo Transplantation of transduced cells myoblasts hepatocytes blood bone marrow fibroblasts skin stem
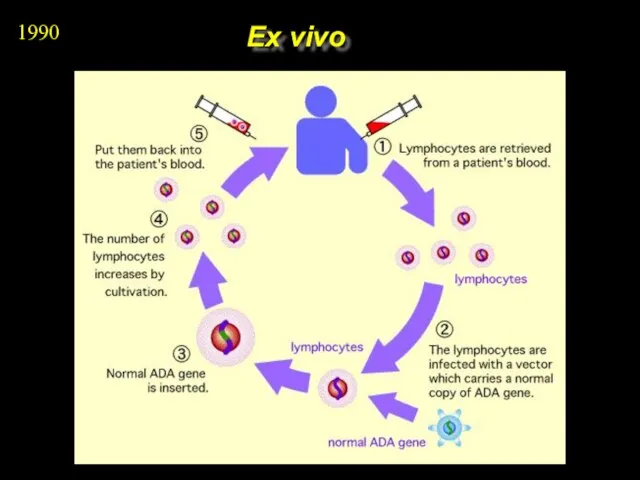
- 7. Ex vivo 1990
- 8. Cells? Which cells? Focus on the patient! Then focus on the disease (cells, tissues…)
- 9. Ex vivo Gene Therapy Lentiviral Hematopoietic Stem Cell Gene Therapy Benefits Metachromatic Leukodystrophy Alessandra Biffi et
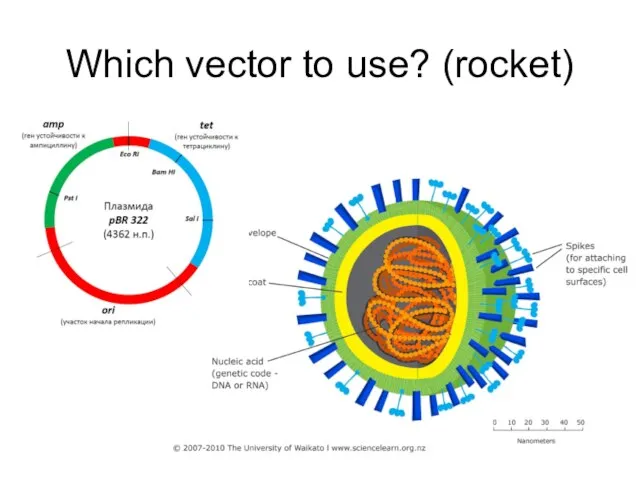
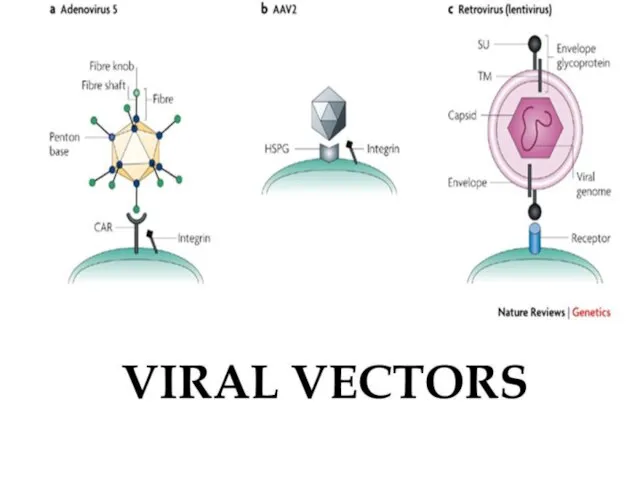
- 10. Which vector to use? (rocket)
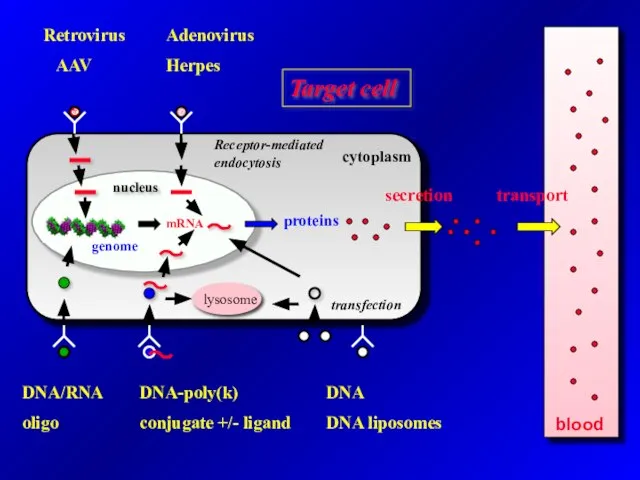
- 11. blood Retrovirus AAV Adenovirus Herpes Target cell DNA/RNA oligo DNA-poly(k) conjugate +/- ligand DNA DNA liposomes
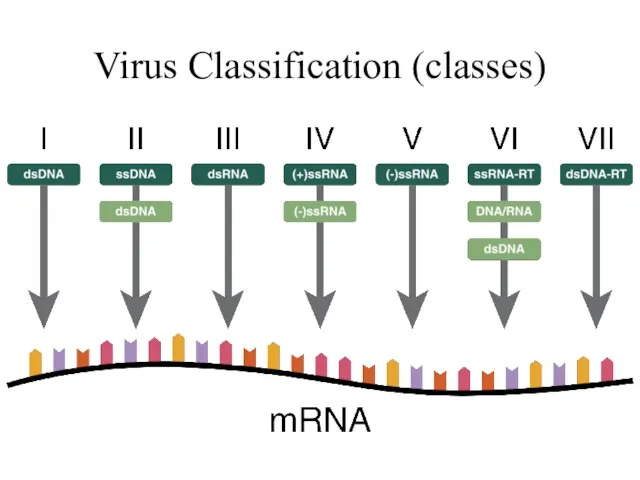
- 12. Virus Classification (classes)
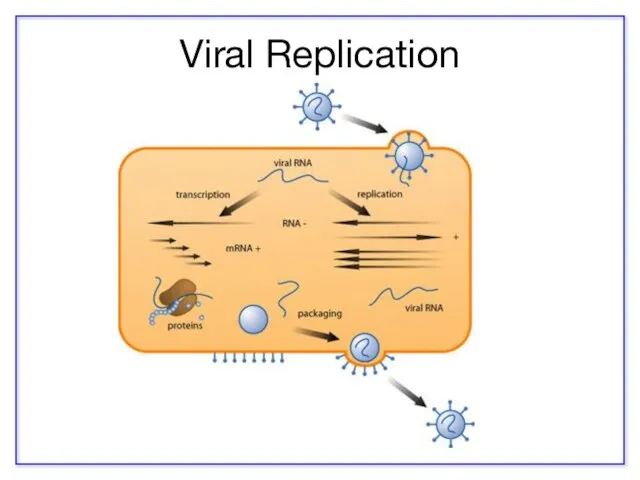
- 13. Viral Replication
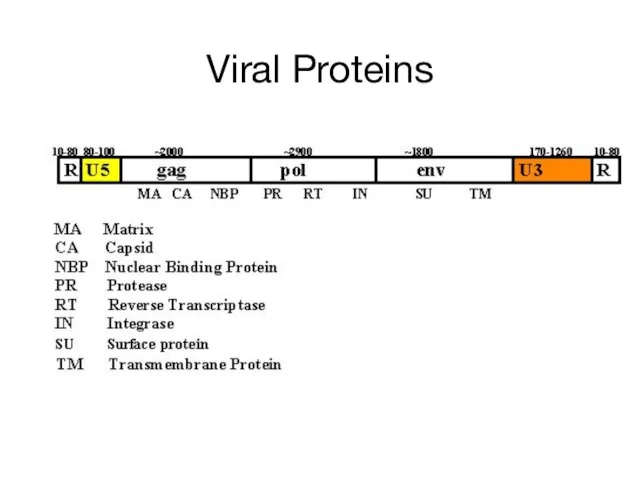
- 14. Viral Proteins
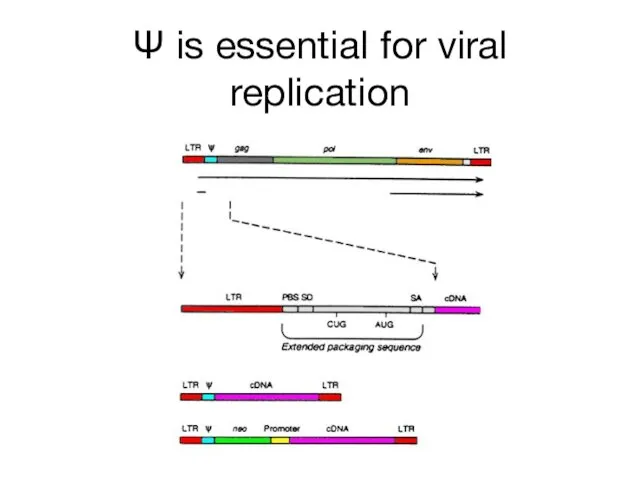
- 15. Ψ is essential for viral replication
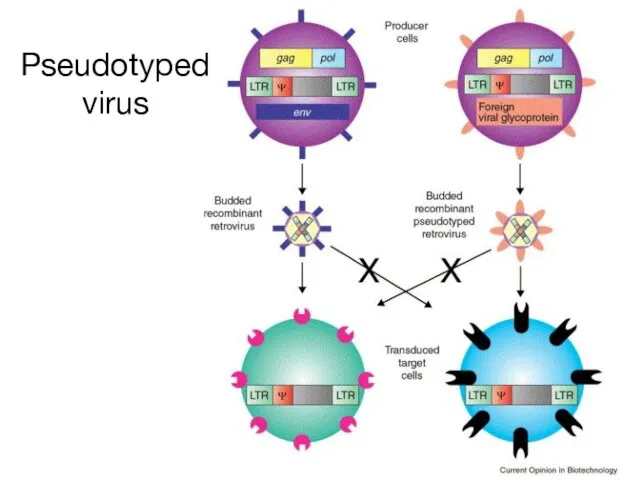
- 16. Pseudotyped virus
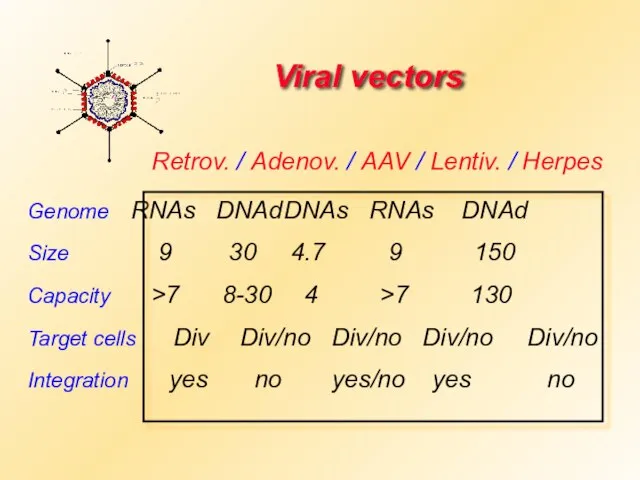
- 17. Viral vectors Genome RNAs DNAd DNAs RNAs DNAd Size 9 30 4.7 9 150 Capacity >7
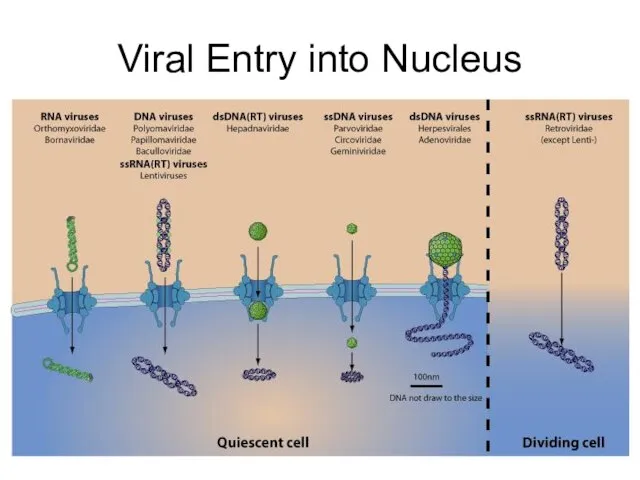
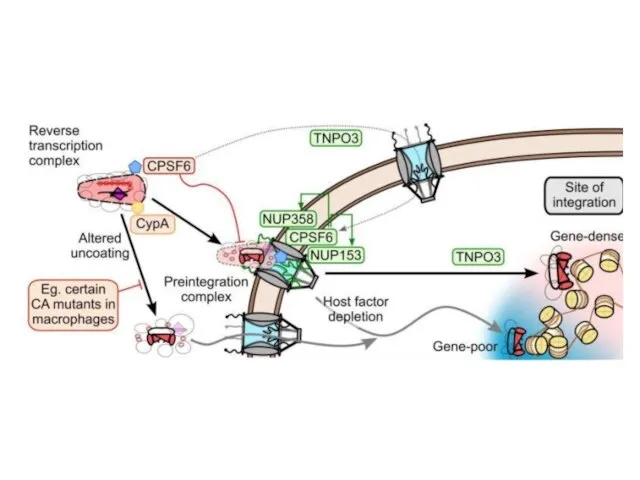
- 19. Viral Entry into Nucleus
- 21. RELEVANT QUESTIONS WHEN CHOSING A VECTOR What disease am I going to target? How long do
- 22. IDEAL VECTOR CANDIDATE (does not yet exist) High titer or concentrations (>108 particles/ml) Method of production
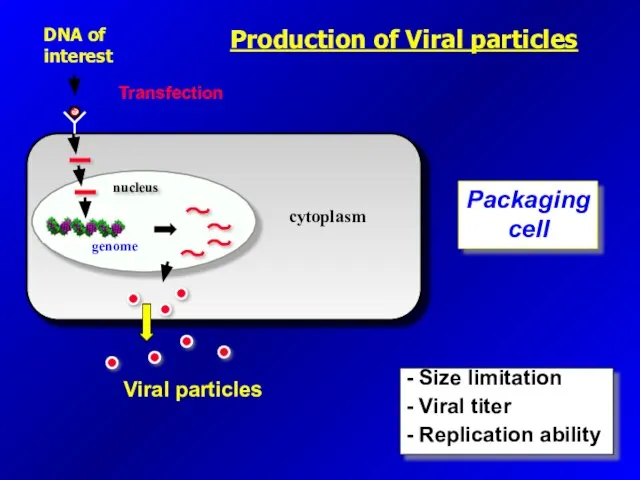
- 23. DNA of interest Transfection Packaging cell Viral particles - Size limitation - Viral titer - Replication
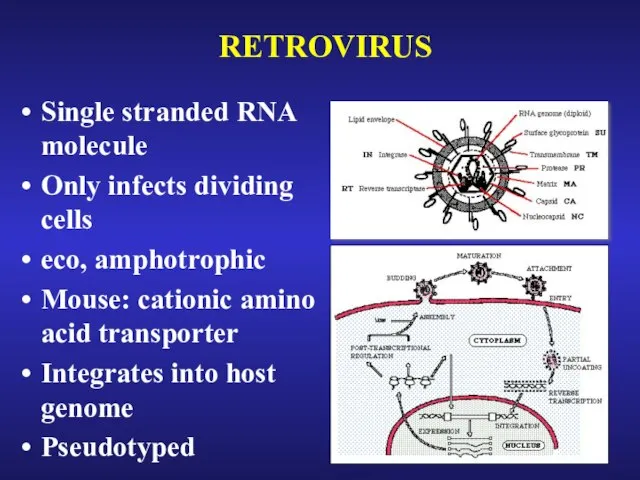
- 24. RETROVIRUS Single stranded RNA molecule Only infects dividing cells eco, amphotrophic Mouse: cationic amino acid transporter
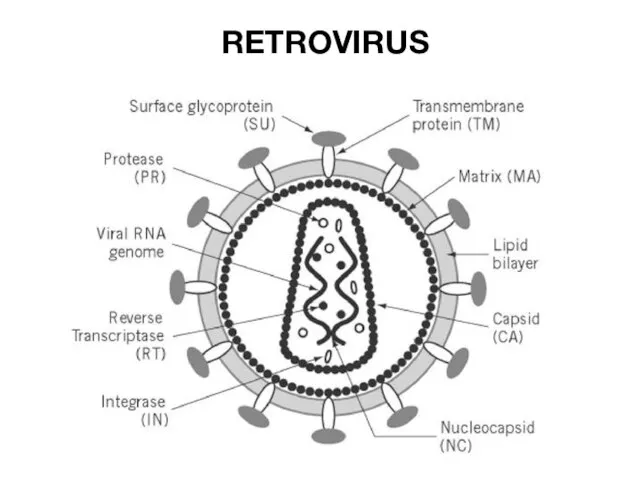
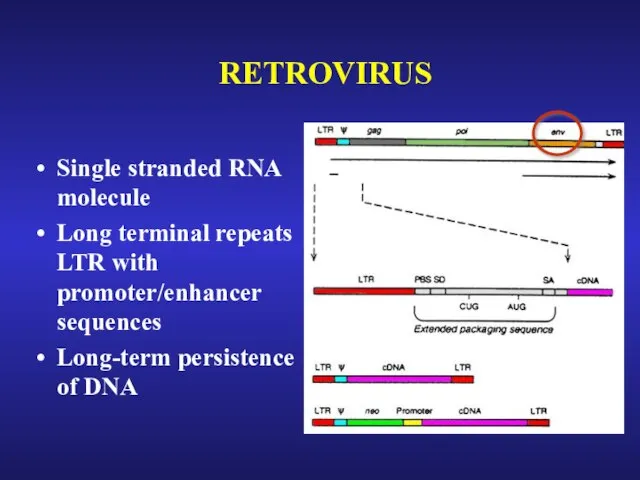
- 25. RETROVIRUS
- 26. RETROVIRUS Single stranded RNA molecule Long terminal repeats LTR with promoter/enhancer sequences Long-term persistence of DNA
- 27. LIMITATIONS OF RETROVIRUS Retroviruses are inactivated by human sera Transgene expression from LTR is often inactivated
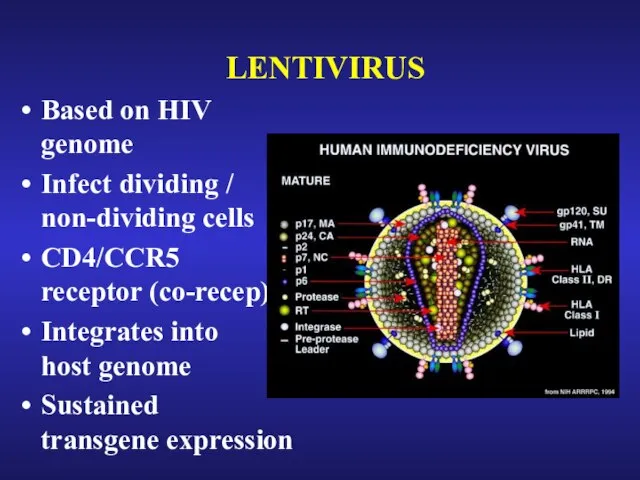
- 28. LENTIVIRUS Based on HIV genome Infect dividing / non-dividing cells CD4/CCR5 receptor (co-recep) Integrates into host
- 29. ADVANTAGES OF LENTIVIRUS Targeting of stem cells Gene expression is sustained, and often sustained through cellular
- 30. LIMITATIONS OF LENTIVIRUS Gene expression is often not as high as with adenovirus Same as retrovirus
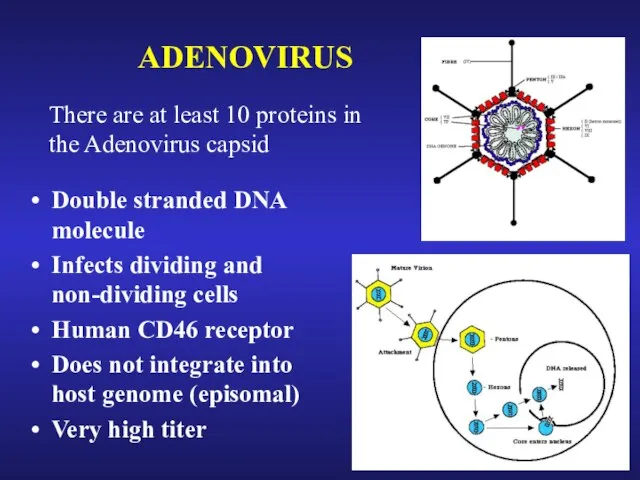
- 31. ADENOVIRUS There are at least 10 proteins in the Adenovirus capsid Double stranded DNA molecule Infects
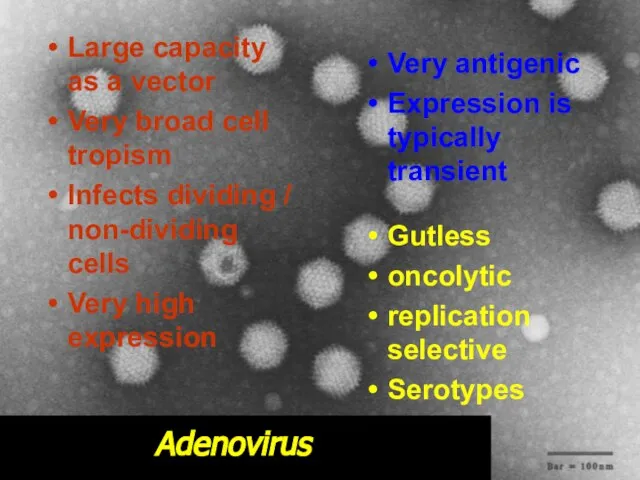
- 32. Adenovirus Large capacity as a vector Very broad cell tropism Infects dividing / non-dividing cells Very
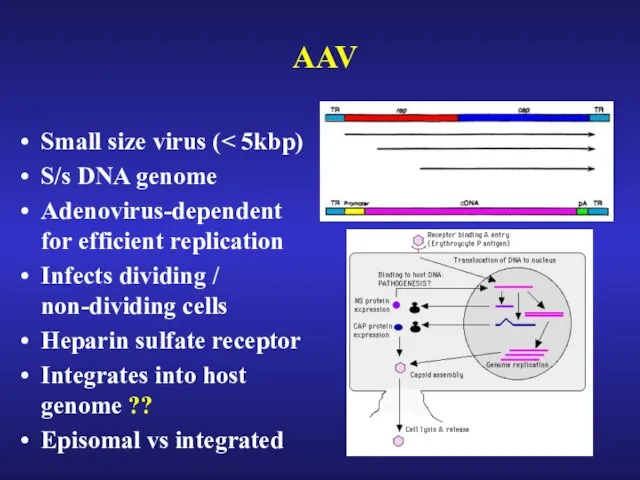
- 33. AAV Small size virus ( S/s DNA genome Adenovirus-dependent for efficient replication Infects dividing / non-dividing
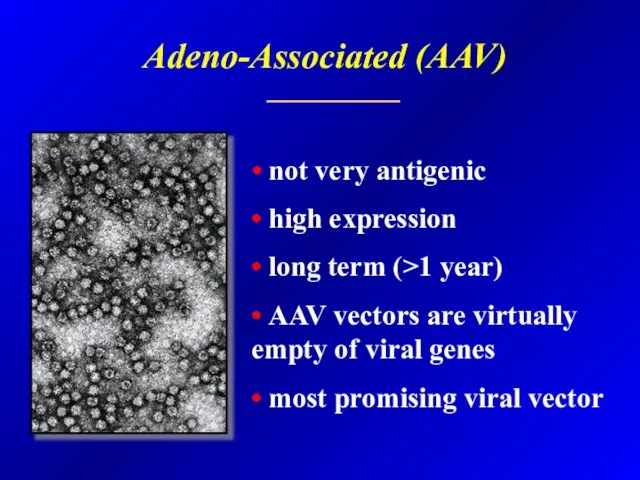
- 34. Adeno-Associated (AAV) • not very antigenic • high expression • long term (>1 year) • AAV
- 35. AAV Lag phase (6 weeks) for max delivery Neutralizing Abs to capsid do not prevent long-term
- 36. HERPES Large size DNA genome (150 kbp) Human neurotropic virus Suitable for targeting the CNS Infects
- 37. HYBRID VECTORS AAV / adenovirus Retrovirus / adenovirus Retrovirus / Herpes
- 38. ALTERNATIVE VIRUS Simbis Poxvirus Vaccinia Baculovirus Sendai Foamy virus SV40…..
- 39. KEY ISSUES Delivery Immune response Logistics Tropism Persistence
- 40. IMMUNITY OF VIRAL VECTORS Delivery Immune response Logistics Tropism Persistence
- 42. Скачать презентацию













 Острый и хронический аппендицит
Острый и хронический аппендицит Определение зависимости коэффициента парного взаимодействия от температуры пар веществ
Определение зависимости коэффициента парного взаимодействия от температуры пар веществ Болезни почек
Болезни почек Болезнь Шейермана-Мау – юношеский кифоз. Болезнь Кальве
Болезнь Шейермана-Мау – юношеский кифоз. Болезнь Кальве Профилактика СПИД и ВИЧ
Профилактика СПИД и ВИЧ Ресвератрол – научный прорыв
Ресвератрол – научный прорыв Флаваноиды
Флаваноиды Chronic gastritis. Хронический гастрит
Chronic gastritis. Хронический гастрит Распространенность ИБС по Старооскольскому округу
Распространенность ИБС по Старооскольскому округу Ортопедическое лечение вкладками
Ортопедическое лечение вкладками Виды проводниковой анестезии
Виды проводниковой анестезии Регуляция тонуса сосудов
Регуляция тонуса сосудов VII жұп берт нерві
VII жұп берт нерві Отеки. Протеинурия беременных
Отеки. Протеинурия беременных Диагностика внутриутробного состояния плода. Кардиотокография при беременности и в родах
Диагностика внутриутробного состояния плода. Кардиотокография при беременности и в родах Мобильное медицинское приложение
Мобильное медицинское приложение Виды томографических исследований
Виды томографических исследований фтиз
фтиз Жіті тыныстың жеткіліксіздігі
Жіті тыныстың жеткіліксіздігі Доклад Тоймухамбетова Ботагоз 719-2 инфекция
Доклад Тоймухамбетова Ботагоз 719-2 инфекция Трудные роды
Трудные роды I этап студенческой олимпиады по хирургии. Абдоминальная хирургия
I этап студенческой олимпиады по хирургии. Абдоминальная хирургия Эмбриогенез человека. Стадии эмбрионального развития. Формирование и развитие зародышевых листков
Эмбриогенез человека. Стадии эмбрионального развития. Формирование и развитие зародышевых листков Природные антибактериальные средства
Природные антибактериальные средства Невропатология. Дизонтогения
Невропатология. Дизонтогения Гиперпластические процессы эндометрия
Гиперпластические процессы эндометрия Значение жидкости для организма
Значение жидкости для организма Острый бронхит
Острый бронхит