Содержание
- 2. [PLEASE ADD YOUR DECLARATION OF INTEREST HERE] The work of GINA is supported only by the
- 3. Current severe asthma guidelines - 2014 Chung et al, ERJ 2014
- 4. Guidelines are costly and time-consuming to develop, and to maintain Typically, guidelines undergo a thorough initial
- 5. The GINA report is not a guideline, but an integrated evidence-based strategy focusing on translation into
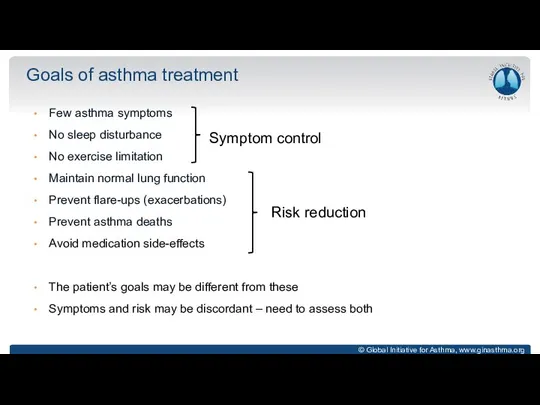
- 6. Goals of asthma treatment Few asthma symptoms No sleep disturbance No exercise limitation Maintain normal lung
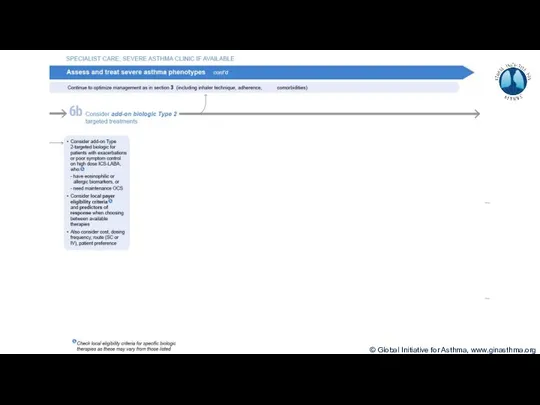
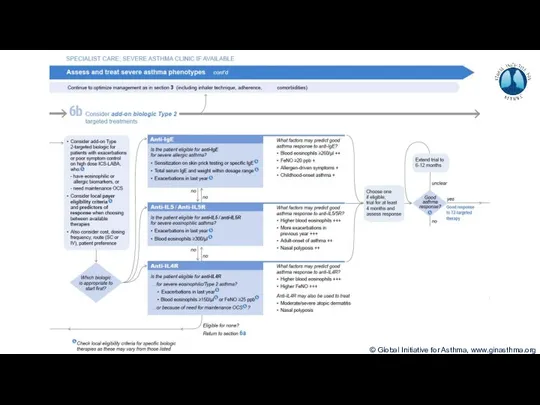
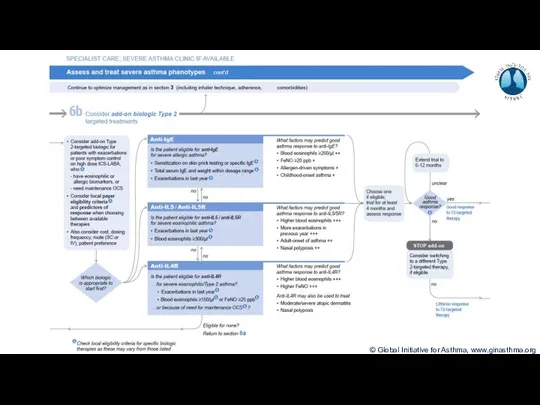
- 7. Terminology Uncontrolled asthma Frequent symptoms and/or flare-ups (exacerbations) Many of these patients may potentially have mild
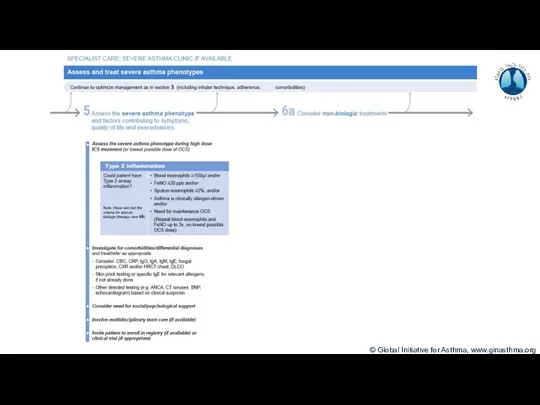
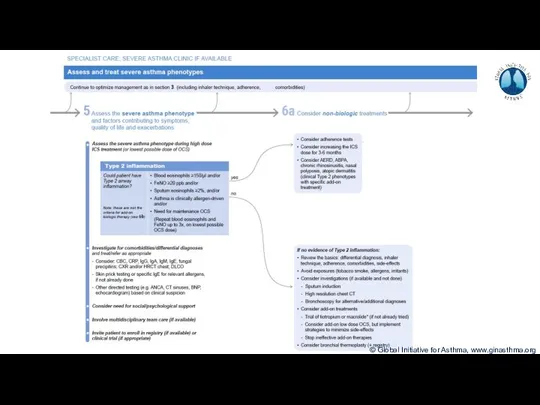
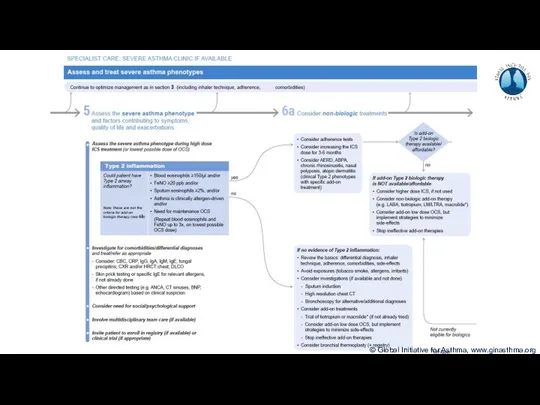
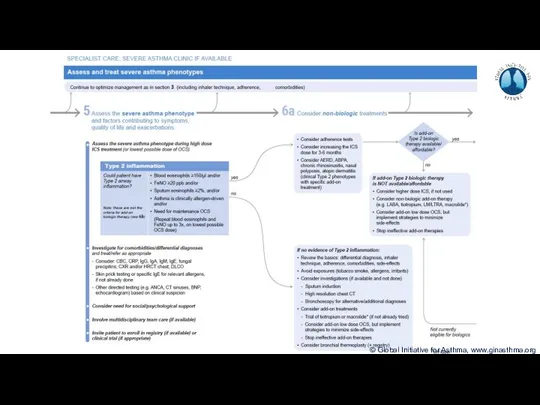
- 8. Phenotype: The observable characteristics of a disease, such as morphology, development, biochemical or physiological properties, or
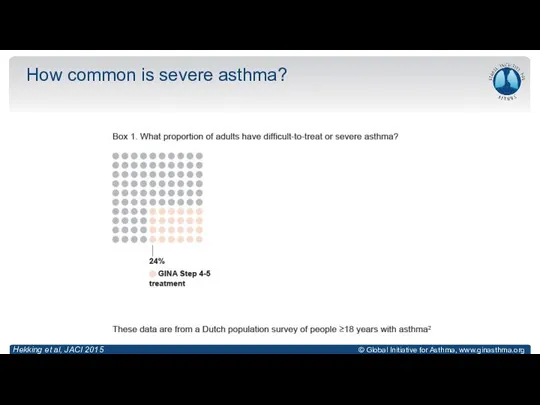
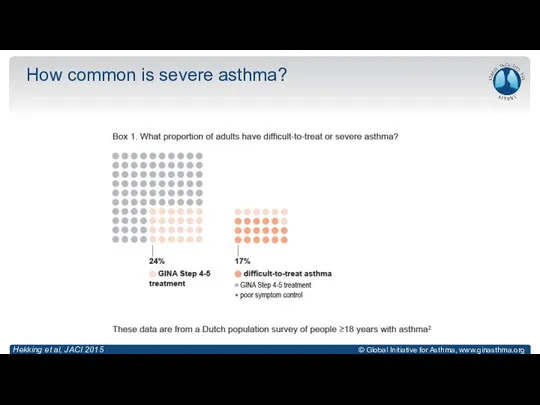
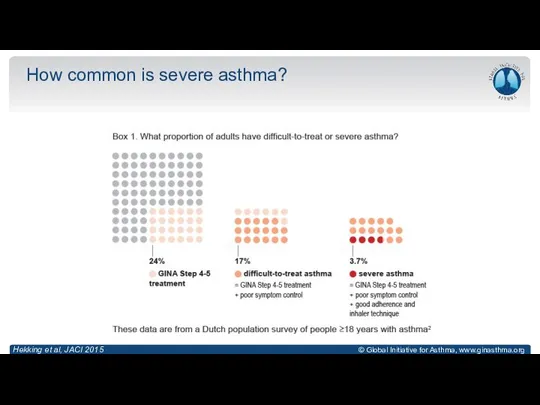
- 9. How common is severe asthma? Hekking et al, JACI 2015
- 10. How common is severe asthma? Hekking et al, JACI 2015
- 11. How common is severe asthma? Hekking et al, JACI 2015
- 12. © Global Initiative for Asthma, www.ginasthma.org
- 13. Tomoko Ichikawa, Clinical Professor of Design, Information Designer, University of Illinois Hugh Musick, Associate Director, Program
- 14. Research: (20+ hours) Familiarized with content area (read papers from prominent authors in the field) Developed
- 15. Decision tree prototype: (60+ hrs, 20 versions) Synthesized content matter, structured into pocket guide outline provided
- 16. © Global Initiative for Asthma, www.ginasthma.org
- 17. © Global Initiative for Asthma, www.ginasthma.org
- 18. © Global Initiative for Asthma, www.ginasthma.org
- 19. © Global Initiative for Asthma, www.ginasthma.org
- 20. © Global Initiative for Asthma, www.ginasthma.org
- 21. © Global Initiative for Asthma, www.ginasthma.org
- 22. © Global Initiative for Asthma, www.ginasthma.org
- 23. © Global Initiative for Asthma, www.ginasthma.org
- 24. © Global Initiative for Asthma, www.ginasthma.org
- 25. © Global Initiative for Asthma, www.ginasthma.org
- 26. © Global Initiative for Asthma, www.ginasthma.org
- 27. © Global Initiative for Asthma, www.ginasthma.org
- 28. © Global Initiative for Asthma, www.ginasthma.org
- 29. © Global Initiative for Asthma, www.ginasthma.org
- 30. © Global Initiative for Asthma, www.ginasthma.org
- 31. © Global Initiative for Asthma, www.ginasthma.org
- 32. © Global Initiative for Asthma, www.ginasthma.org
- 33. © Global Initiative for Asthma, www.ginasthma.org
- 34. © Global Initiative for Asthma, www.ginasthma.org
- 35. © Global Initiative for Asthma, www.ginasthma.org
- 36. © Global Initiative for Asthma, www.ginasthma.org
- 37. © Global Initiative for Asthma, www.ginasthma.org
- 38. © Global Initiative for Asthma, www.ginasthma.org
- 40. Скачать презентацию
![[PLEASE ADD YOUR DECLARATION OF INTEREST HERE] The work of GINA is](/_ipx/f_webp&q_80&fit_contain&s_1440x1080/imagesDir/jpg/1053764/slide-1.jpg)





















 Патологические рефлексы
Патологические рефлексы Энцефалограмма
Энцефалограмма The Survey of digestive health Acrjss Europe
The Survey of digestive health Acrjss Europe Язвы роговицы
Язвы роговицы Мерцательная аритмия
Мерцательная аритмия Лейкоз. Визначення хвороби
Лейкоз. Визначення хвороби Эффективность применения двучелюстных шин Тигерштедта по сравнению с фиксацией остеосинтезом
Эффективность применения двучелюстных шин Тигерштедта по сравнению с фиксацией остеосинтезом Неврологическая симптоматика болезни Уиппла
Неврологическая симптоматика болезни Уиппла Связь заболеваний пародонта и риска преждевременных родов и низкого веса новорожденного
Связь заболеваний пародонта и риска преждевременных родов и низкого веса новорожденного Спорынья (Claviceps). Фармакологические свойства
Спорынья (Claviceps). Фармакологические свойства Лабораторные методы в дифференциальной диагностике пневмоний различной этиологии
Лабораторные методы в дифференциальной диагностике пневмоний различной этиологии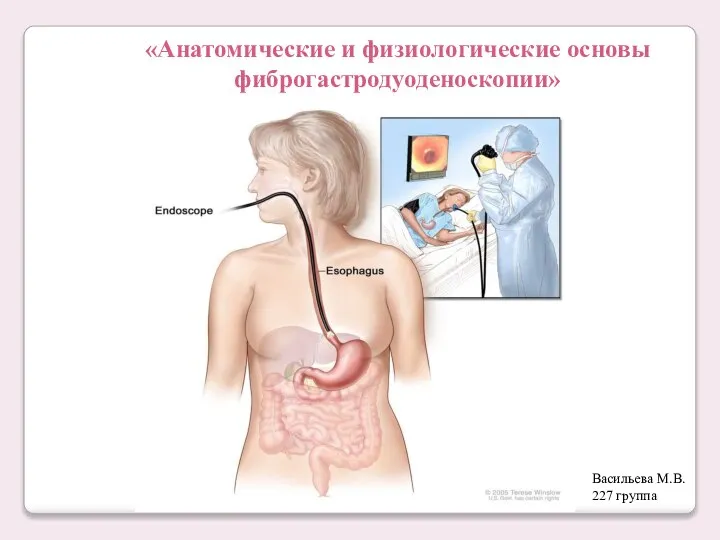 Анатомические и физиологические основы фиброгастродуоденоскопии
Анатомические и физиологические основы фиброгастродуоденоскопии Здоровье глаз
Здоровье глаз Я и моя будущая профессия
Я и моя будущая профессия Заболевания шеи и щитовидной железы
Заболевания шеи и щитовидной железы Tolerance, autoimmunity, allogenicity
Tolerance, autoimmunity, allogenicity Оценка формирования моторики с использованием Системы оценки больших моторных функций GMFCS
Оценка формирования моторики с использованием Системы оценки больших моторных функций GMFCS Бронхиалды демікпенің емі
Бронхиалды демікпенің емі Дифференциальная диагностика ДПН
Дифференциальная диагностика ДПН Иммунитет. Воспаление
Иммунитет. Воспаление Печень. Общий вид печени
Печень. Общий вид печени Оказание медицинской помощи при поражениях токсичными химическими веществами общеядовитого действия
Оказание медицинской помощи при поражениях токсичными химическими веществами общеядовитого действия Особенности применения различных лекарственных форм у детей раннего возраста
Особенности применения различных лекарственных форм у детей раннего возраста Артериальная гипертензия
Артериальная гипертензия Техника удаления зубов на верхней челюсти, инструменты
Техника удаления зубов на верхней челюсти, инструменты Правовое регулирование трансплантации органов и тканей и донорства
Правовое регулирование трансплантации органов и тканей и донорства Анализ эффективности ветеринарных мероприятий по профилактике диспепсии молодняка крупнорогатого скота
Анализ эффективности ветеринарных мероприятий по профилактике диспепсии молодняка крупнорогатого скота Роль наддесневых зубных камней в развитии пародонтоза
Роль наддесневых зубных камней в развитии пародонтоза