Слайд 2Abstract
Interindividual variability in methadone disposition remains unexplained, and methadone accidental overdose

in pain therapy is a significant public health problem. Cytochrome P4502B6 (CYP2B6) is the principle determinant of clinical methadone elimination. The CYP2B6 gene is highly polymorphic, with several variant alleles. CYP2B6.6, the protein encoded by the CYP2B6*6 polymorphism, deficiently catalyzes methadone metabolism in vitro. This investigation determined the influence of CYP2B6*6, and other allelic variants encountered, on methadone concentrations, clearance, and metabolism.
Слайд 3Methadone
is a long-duration opioid
for acute, chronic, perioperative, neuropathic, and cancer pain
Methadone is

typically a racemic mixture
R-methadone primarily confers the μ-opioid receptor activity
both enantiomers act at N-methyl-d-aspartate receptors
! In 2009, methadone accounted for only 2% of prescriptions, but 30% of prescription painkiller deaths !
Understanding methadone disposition is important for reducing adverse events
Слайд 4Methadone is cleared by:
Hepatic cytochrome P450 (CYP)-catalyzed N-demethylation
to inactive

2-ethyl- 1,5-dimethyl-3,3-diphenylpyrrolidine (EDDP)
some urinary excretion of unchanged drug
Слайд 5The CYP2B6 gene in relation to Methadone.
Highly polymorphic (38 variant alleles identified)
CYP2B6*6
is the most common and clinically significant variant allele
The hypothesis:
CYP2B6*6 heterozygotes or homozygotes would have reduced metabolism and clearance.
Is to evaluate other less common genotypic variants, when encountered.
Слайд 6Materials and Methods
Inclusion criteria were:
18- to 50-yr-old normal healthy volunteers
good general health

without remarkable medical conditions
and within 30% of ideal body weight (body mass index < 33 kg/m2 )
Exclusion criteria were:
a history of hepatic or renal disease
use of prescription or nonprescription medications, herbals or foods known to be metabolized by or affect the activity of CYP2B6
known history of drug or alcohol addiction
Слайд 7Potential subjects provided a venous blood sample, and genomic DNA was isolated

from peripheral blood leukocytes by using the Gentra Puregene Blood Kit
Genotyping was performed by the Genome Technology Access Center at Washington University in St. Louis by using the Fluidigm BioMark System
Genotyping results were then used to invite subject participation and create target cohorts of 20 subjects each with CYP2B6*1/*1, CYP2B6*1/*6, and CYP2B6*6/*6 genotypes
Слайд 8Subjects were instructed to refrain from:
alcohol for 48h before and during the

study day
caffeine-containing beverages on the study day
oranges, grapefruit, or apples or their juices for 5 days before and throughout the 96-h study period
food/liquids after midnight the day before methadone administration
nonstudy medications (including over the counter and/or herbal) for 3 days before the study day, without previous approval
Слайд 10Data and Statistical Analysis
Pharmacokinetic data were analyzed by using noncompartmental methods
Results
are reported as the arithmetic mean ± SD
The primary outcome measure was methadone metabolism, measured as plasma EDDP/methadone area under the concentration– time curve (AUC0–96) ratio and EDDP formation clearance.
Secondary outcomes included methadone peak plasma concentration, exposure (plasma AUC∞)
Слайд 11Results
Allele frequencies are consistent with the previous reports
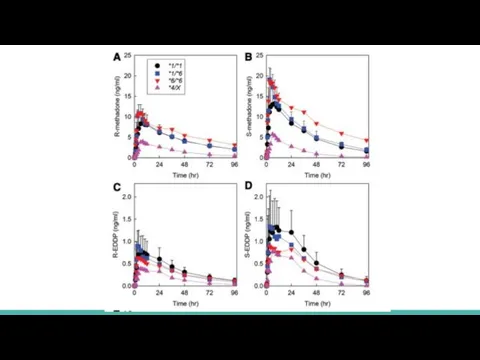
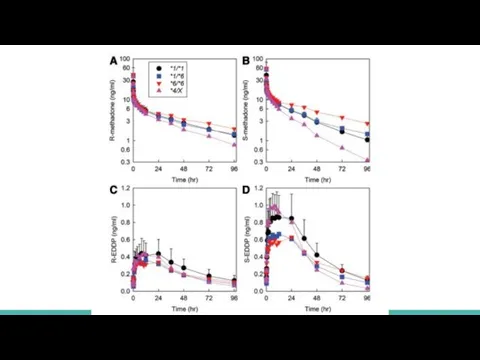
Plasma methadone and EDDP enantiomer

concentrations are shown for oral (fig. 1) and IV (fig. 2) methadone, for the three major genotype groups (CYP2B6*1/*1, CYP2B6*1/*6, and CYP2B6*6/*6) and for *4 carriers (CYP2B6*1/*4 and CYP2B6*4/*6, shown together as CYP2B6*4/X)
Genotype influence was greater for oral than IV dosing and for S- than R-methadone
Слайд 12 For oral methadone, average plasma exposure
FOR S-METHADONE
in CYP2B6*1/*1 was 620 ±

230 ng/ml-h
in CYP2B6*1/*6 was 734 ± 245 ng/ml-h
in CYP2B6*6/*6 was 1,242 ± 801 ng/ml-h
FOR R-METHADONE
in CYP2B6*1/*1 was 578 ± 205 ng/ml-h
in CYP2B6*1/*6 was 615 ± 172 ng/ml-h
in CYP2B6*6/*6 was 898 ± 507 ng/ml-h
CYP2B6*4/X subjects was 155 ± 45 and 177 ± 48 for S- and R-methadone, respectively
Слайд 14For IV methadone
FOR S-METHADONE
in CYP2B6*1/*1 was 447 ± 85
in CYP2B6*1/*6 was 513

± 171
in CYP2B6*6/*6 was 801 ± 464
FOR R-METHADONE
in CYP2B6*1/*1 was 430 ± 131
in CYP2B6*1/*6 was 429 ± 135
in CYP2B6*6/*6 was 570 ± 281
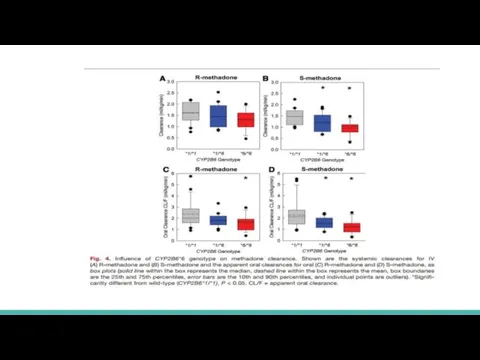
Слайд 16Hepatic clearance (ml kg−1 min−1) was significantly less in CYP2B6*6/*6 compared with

that of CYP2B6*1/*1 subjects for S-methadone (0.8 ±0.4 and 1.3±0.3) but not R-methadone (1.0 ±0.3 and 1.3±0.3)
Methadone N-demethylation was significantly less in CYP2B6*6 carriers, particularly homozygotes, and apparently greater in CYP2B6*4 carriers
S-methadone systemic clearance (ml kg−1 min−1) in CYP2B6*1/*6 and CYP2B6*6/*6 subjects (1.2 ± 0.4 and 0.96 ±0.33, respectively) was significantly less than in CYP2B6*1 homozygotes (1.5 ±0.3)
R-methadone clearances in CYP2B6*6 carriers were not significantly different from CYP2B6*1/*1 subjects
In contrast, R- and S-methadone systemic clearances (2.4 ± 0.7 and 2.7 ± 0.9) and apparent oral clearances (7.4 ± 3.8 and 8.6 ± 3.2) were numerically greater in CYP2B6*4/X subjects than in CYP2B6*1/*1 subjects.
Слайд 18Conclusion
Methadone disposition was stereoselective, with greater initial exposure to S-methadone
Plasma methadone concentration

change was diminished in CYP2B6*6 allele carriers and accentuated in CYP2B6*4 carriers.
CYP2B6*6 allele carriers, particularly homozygotes, had higher methadone concentrations and slower elimination, whereas CYP2B6*4 carriers had lower concentrations and faster elimination.
Слайд 19Discussion
Allelic influences on methadone concentrations were caused by differences in clearance
CYP2B6 genetic
influence on methadone metabolism and clearance further highlights and reinforces CYP2B6 as the predominant CYP responsible for clinical methadone elimination.
It is now established, after recognizing CYP2B6 as a major catalyst of methadone metabolism in vitro, 28,44–47 and from numerous clinical drug interaction studies, that CYP2B6, not CYP3A4, is the principle determinant of methadone elimination.
These results provide a mechanistic understanding for interindividual variability in methadone elimination and may have clinical implications for genetically based improvements in methadone dosing, effectiveness, and toxicity














 Анализ клинического случая и обсуждение плана лечения
Анализ клинического случая и обсуждение плана лечения Распространение вирусных инфекций
Распространение вирусных инфекций Интеграция и регуляция метаболизма
Интеграция и регуляция метаболизма анаэробные инфекции
анаэробные инфекции Жая етіне қойылатын жалпы талаптар. СТ РК 1303-2004
Жая етіне қойылатын жалпы талаптар. СТ РК 1303-2004 Гипергликемия. Что такое гипергликемия?
Гипергликемия. Что такое гипергликемия? Психотипы пациентов и способы взаимодествия с ними
Психотипы пациентов и способы взаимодествия с ними Периферический увеит
Периферический увеит Острый и подострый эндокардит (инфекционный эндокардит). Лечение
Острый и подострый эндокардит (инфекционный эндокардит). Лечение Объемные образования позвоночника спинного мозга
Объемные образования позвоночника спинного мозга Системная красная волчанка (СКВ)
Системная красная волчанка (СКВ) Физиология обмена кальция
Физиология обмена кальция Практические навыки. Повязки
Практические навыки. Повязки Синдром диссеминированного внутрисосудистого свертывания крови (ДВС)
Синдром диссеминированного внутрисосудистого свертывания крови (ДВС) PBSerum Halyna Krinska basic
PBSerum Halyna Krinska basic Адренергические средства
Адренергические средства Агнозии. Зрительные агнозии
Агнозии. Зрительные агнозии Гиппократ анты
Гиппократ анты Химия для здоровья человека
Химия для здоровья человека Инфаркт миокарда
Инфаркт миокарда Новий санітарний регламент
Новий санітарний регламент Дифференциальная диагностика и принципы лечения синдрома Корнелии Де Ланге, а также синдром Нунан, синдром Рубинштейна-Тейби
Дифференциальная диагностика и принципы лечения синдрома Корнелии Де Ланге, а также синдром Нунан, синдром Рубинштейна-Тейби Сулейманов Омар
Сулейманов Омар Prezentatsia_po_OBZh_7_klass_Biologo-sotsialnye_chrezvychaynye_situatsii
Prezentatsia_po_OBZh_7_klass_Biologo-sotsialnye_chrezvychaynye_situatsii Кровотечения. Гемостаз
Кровотечения. Гемостаз Female anopheles. Malaria and lymphatic filariasis
Female anopheles. Malaria and lymphatic filariasis Психологиялық зерттеу әдістері
Психологиялық зерттеу әдістері Ботулизм (Botulismus)
Ботулизм (Botulismus)