Содержание
- 2. Alkali Metals Electron structure and reactivity Physical properties Summary activities Reactions Uses Contents
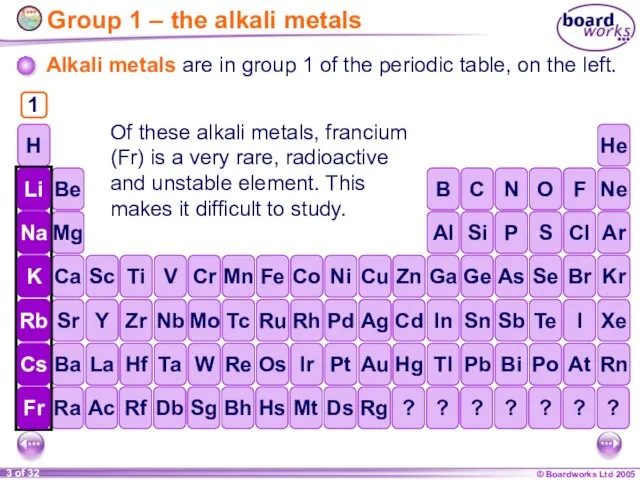
- 3. Group 1 – the alkali metals Alkali metals are in group 1 of the periodic table,
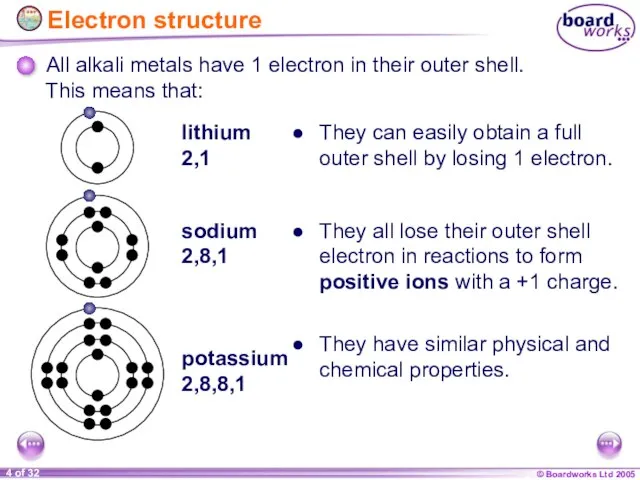
- 4. Electron structure All alkali metals have 1 electron in their outer shell. lithium 2,1 sodium 2,8,1
- 5. Electron structure and reactivity The reactivity of alkali metals increases down the group. What is the
- 6. Reactivity of the alkali metals
- 7. Alkali Metals Electron structure and reactivity Physical properties Summary activities Reactions Uses Contents
- 8. General properties Alkali metals are different to typical (transition) metals, such as iron and copper. Unlike
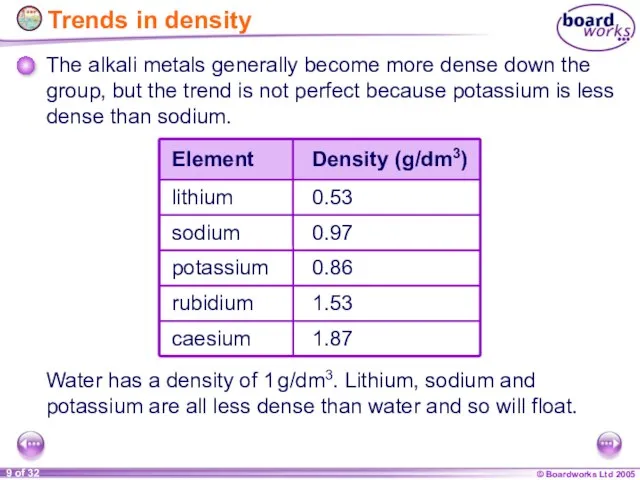
- 9. Trends in density The alkali metals generally become more dense down the group, but the trend
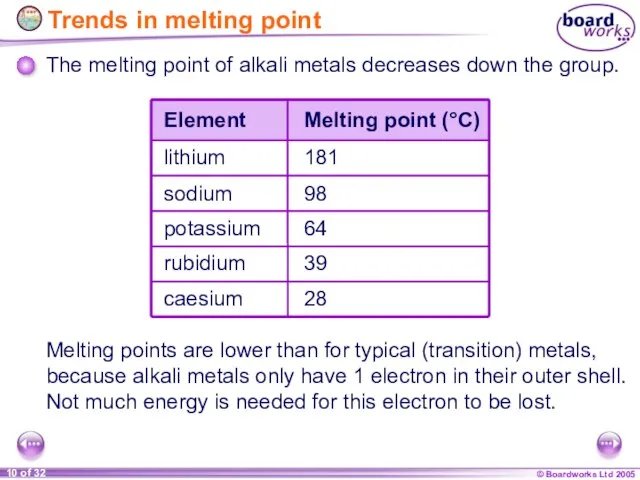
- 10. Trends in melting point The melting point of alkali metals decreases down the group. Melting points
- 11. Alkali Metals Electron structure and reactivity Physical properties Summary activities Reactions Uses Contents
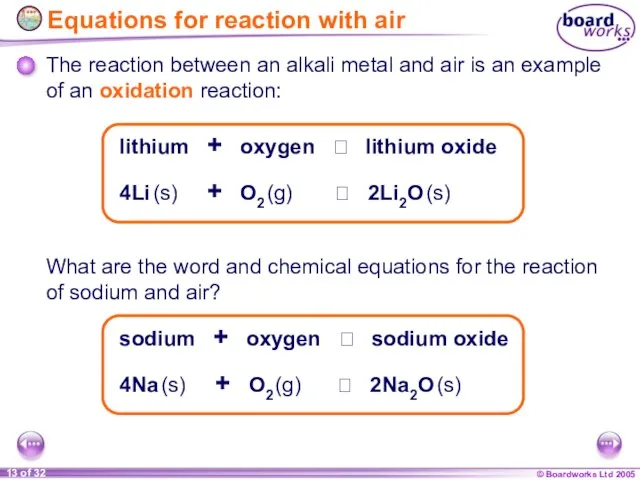
- 12. Reactions with air All alkali metals react with air to form metal oxides. This produces a
- 13. 4Li (s) + O2 (g) 2Li2O (s) What are the word and chemical equations for
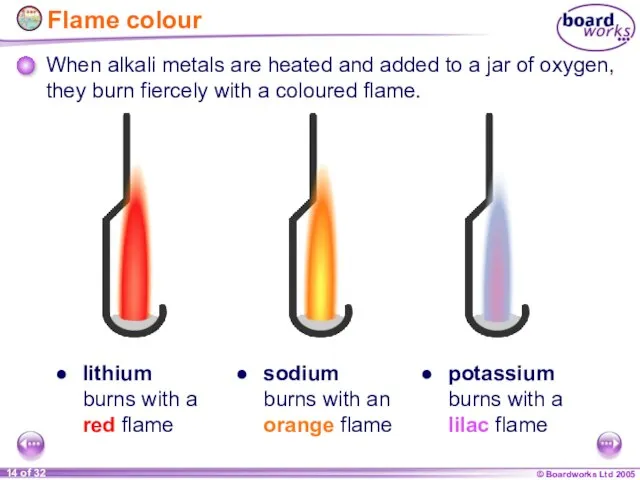
- 14. Flame colour When alkali metals are heated and added to a jar of oxygen, they burn
- 15. Alkali metals and water How do alkali metals react with water?
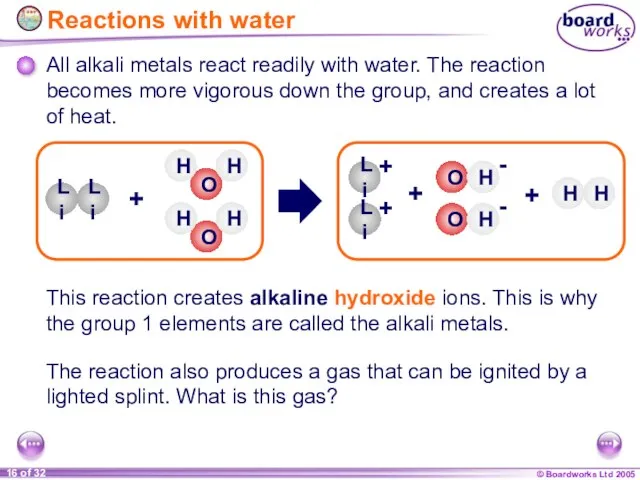
- 16. All alkali metals react readily with water. The reaction becomes more vigorous down the group, and
- 17. Reactivity of alkali metals with water
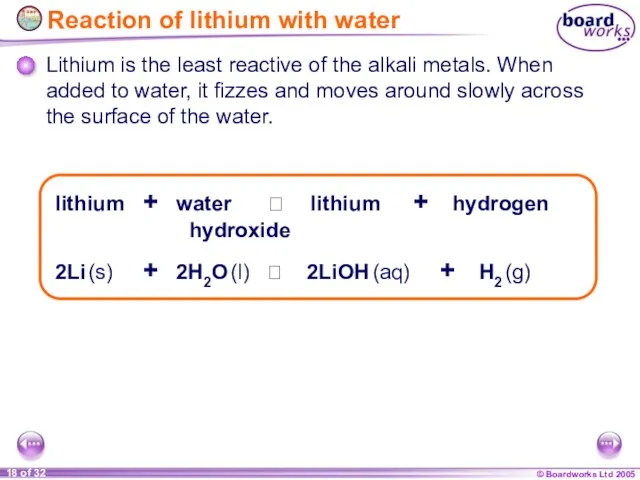
- 18. Reaction of lithium with water 2Li (s) + 2H2O (l) 2LiOH (aq) + H2 (g)
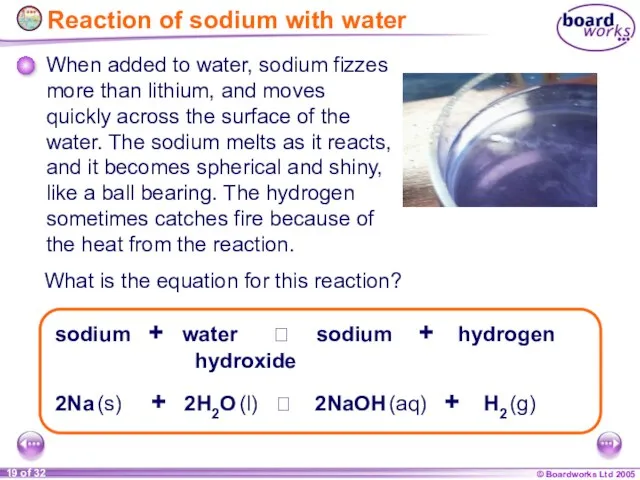
- 19. Reaction of sodium with water 2Na (s) + 2H2O (l) 2NaOH (aq) + H2 (g)
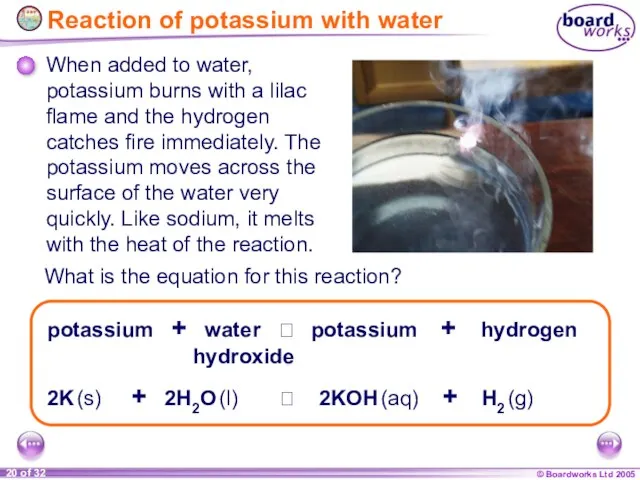
- 20. Reaction of potassium with water When added to water, potassium burns with a lilac flame and
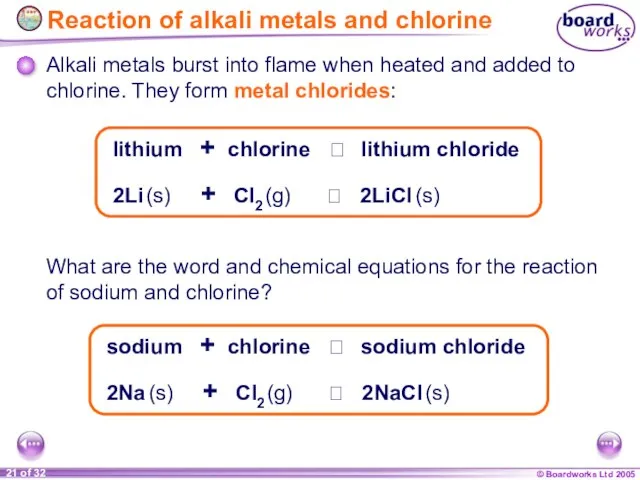
- 21. Alkali metals burst into flame when heated and added to chlorine. They form metal chlorides: Reaction
- 22. True or false?
- 23. Alkali Metals Electron structure and reactivity Physical properties Summary activities Reactions Uses Contents
- 24. Uses of lithium medical treatment – lithium carbonate is sometimes used to treat mental illnesses such
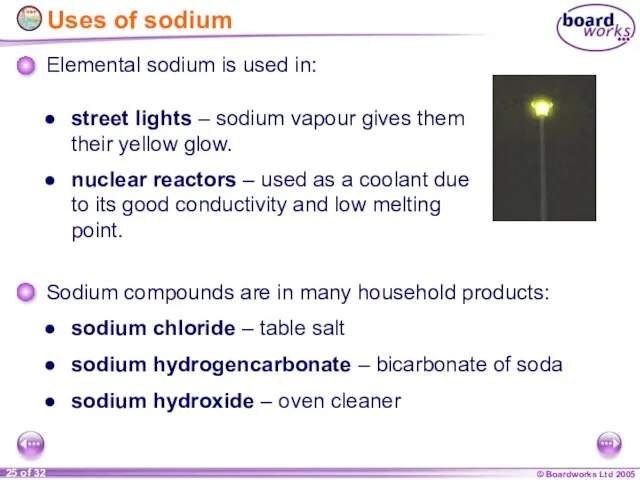
- 25. Uses of sodium sodium chloride – table salt street lights – sodium vapour gives them their
- 26. Uses of potassium Potassium compounds are used in: fertilizers – potassium is an essential element for
- 27. Alkali Metals Electron structure and reactivity Physical properties Summary activities Reactions Uses Contents
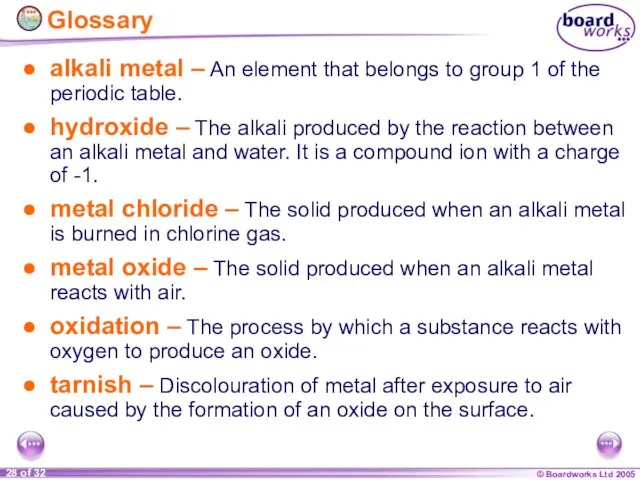
- 28. Glossary alkali metal – An element that belongs to group 1 of the periodic table. hydroxide
- 29. Anagrams
- 30. Completing alkali metal equations
- 31. Comparing reactivity with water
- 33. Скачать презентацию








 Системы лояльности: современные тенденции развития
Системы лояльности: современные тенденции развития Теорема Виета доказательство
Теорема Виета доказательство Словообразовательные гнёзда полисемантичных имён существительных в русском и белорусском языках
Словообразовательные гнёзда полисемантичных имён существительных в русском и белорусском языках СМАЗКИ КАНАТНЫЕ
СМАЗКИ КАНАТНЫЕ Приемы рисования геометрических фигур
Приемы рисования геометрических фигур Metal-Insulator-Semiconductor and Metal-Insulator-Metal Structures
Metal-Insulator-Semiconductor and Metal-Insulator-Metal Structures "Я ЛЮБЛЮ ТЕБЯ,РОССИЯ!" Игра "Звездный час" (для учащихся 3-4классов)
"Я ЛЮБЛЮ ТЕБЯ,РОССИЯ!" Игра "Звездный час" (для учащихся 3-4классов) Три кита в музыке
Три кита в музыке Сбор изображений для тренировки системы распознавания номеров машин
Сбор изображений для тренировки системы распознавания номеров машин Презентация на тему Состав ядра. Ядерные силы (11 класс)
Презентация на тему Состав ядра. Ядерные силы (11 класс) Понятие мотивации. Мотивация по Риссу. Нейрологические уровни Дилтса. Модель ценностей Грейвза
Понятие мотивации. Мотивация по Риссу. Нейрологические уровни Дилтса. Модель ценностей Грейвза Финансовая политика РФ
Финансовая политика РФ Дециметр
Дециметр Материки и океаны
Материки и океаны Конституционное право - ведущая отрасль в правовой системе Российской Федерации. Лекция 1
Конституционное право - ведущая отрасль в правовой системе Российской Федерации. Лекция 1 Александр Родченко
Александр Родченко Спектры.Спектральный анализОткрытый урок
Спектры.Спектральный анализОткрытый урок Лепка фигуры человека
Лепка фигуры человека ОПСиП_ Семенова ПО-3
ОПСиП_ Семенова ПО-3 Градусная сеть на глобусе и географической карте
Градусная сеть на глобусе и географической карте Международный Юридический институт приглашает всех желающих на День Открытых дверей!
Международный Юридический институт приглашает всех желающих на День Открытых дверей! Страхование непредвиденных расходов автовладельцев полис «РЕСОавто ПОМОЩЬ»
Страхование непредвиденных расходов автовладельцев полис «РЕСОавто ПОМОЩЬ» Бюджет доходов и расходов БДР/P&L
Бюджет доходов и расходов БДР/P&L Лексика
Лексика אילו המצאות חדשות הומצאו בישראל ובעולם ?במאה ה?21 -במה תרומתם לאנושות
אילו המצאות חדשות הומצאו בישראל ובעולם ?במאה ה?21 -במה תרומתם לאנושות Главные и второстепенные члены предложения
Главные и второстепенные члены предложения Основные причины ухудшения зрения школьника
Основные причины ухудшения зрения школьника Качество и качества Власти: восприятие населения
Качество и качества Власти: восприятие населения