Содержание
- 2. 11.1.3.1 understand and be able to work with a shell model of the atom: shell, sub-shell,
- 3. Success criteria explain the shell - subshell - orbital structure of the atom and relate it
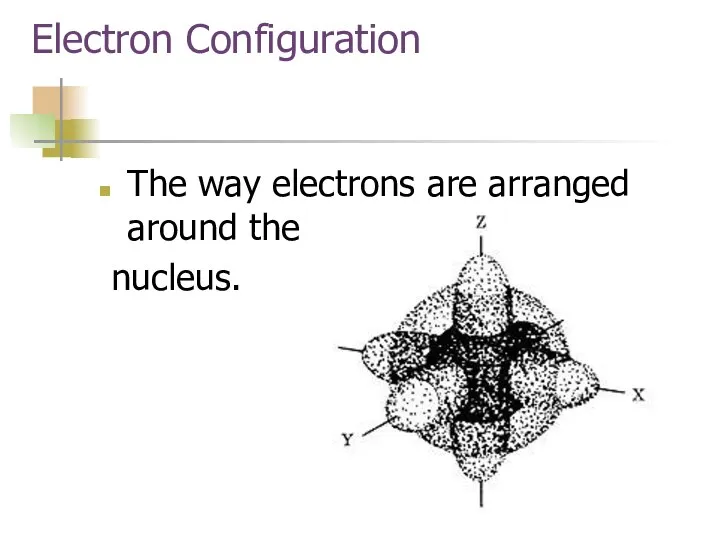
- 4. Electron Configuration The way electrons are arranged around the nucleus.
- 5. Quantum Mechanical Model 1920’s Werner Heisenberg (Uncertainty Principle) Louis de Broglie (electron has wave properties) Erwin
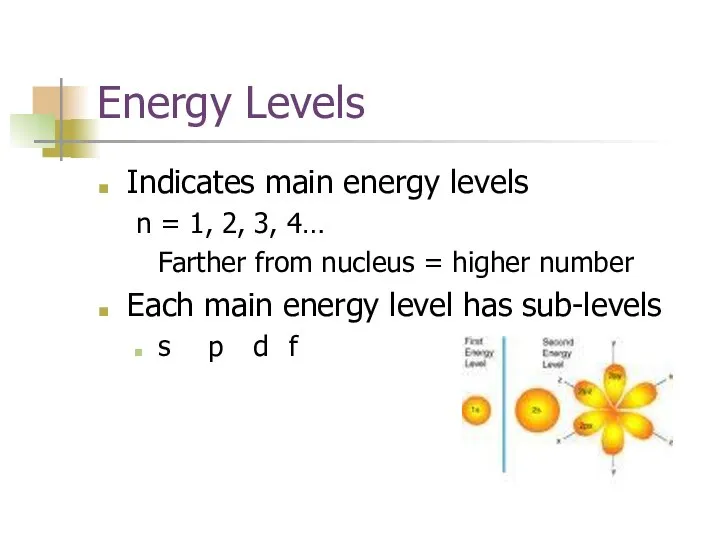
- 6. Energy Levels Indicates main energy levels n = 1, 2, 3, 4… Farther from nucleus =
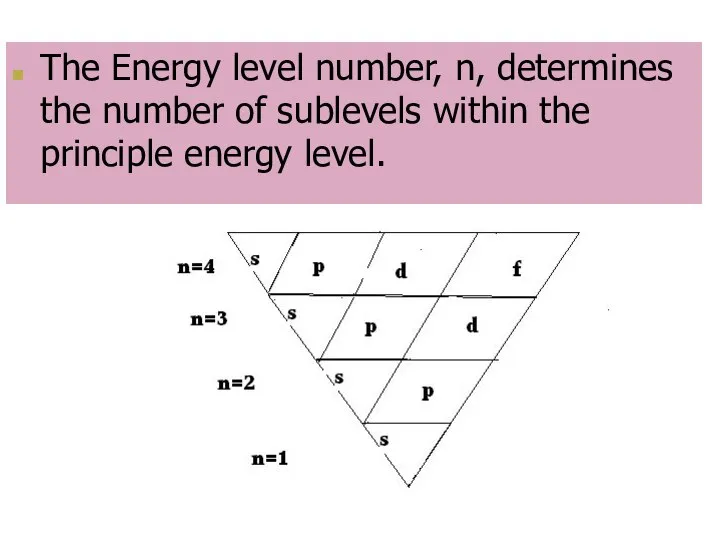
- 7. The Energy level number, n, determines the number of sublevels within the principle energy level.
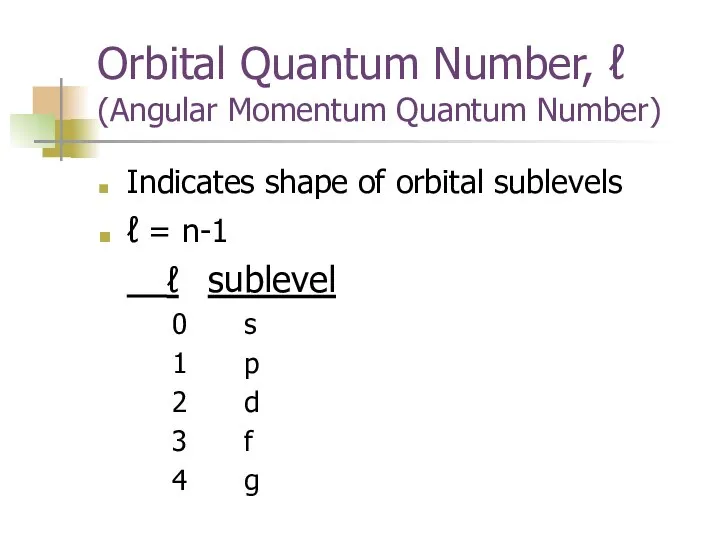
- 8. Orbital Quantum Number, ℓ (Angular Momentum Quantum Number) Indicates shape of orbital sublevels ℓ = n-1
- 9. Orbital The space where there is a high probability that it is occupied by a pair
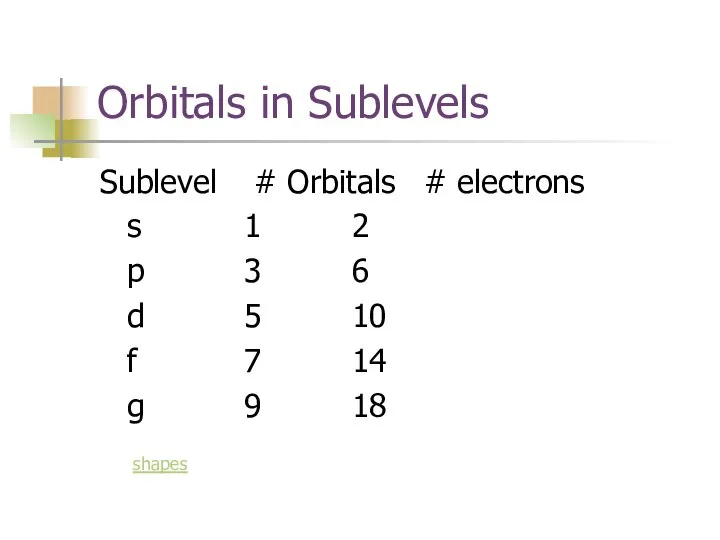
- 10. Orbitals in Sublevels Sublevel # Orbitals # electrons s 1 2 p 3 6 d 5
- 11. Three rules are used to build the electron configuration: Aufbau principle Pauli Exclusion Principle Hund’s Rule
- 12. Aufbau Principle Electrons occupy orbitals of lower energy first.
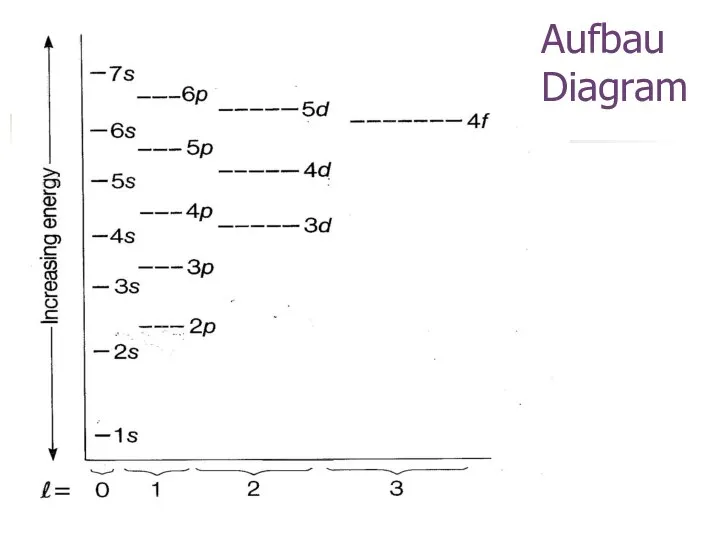
- 13. Aufbau Diagram
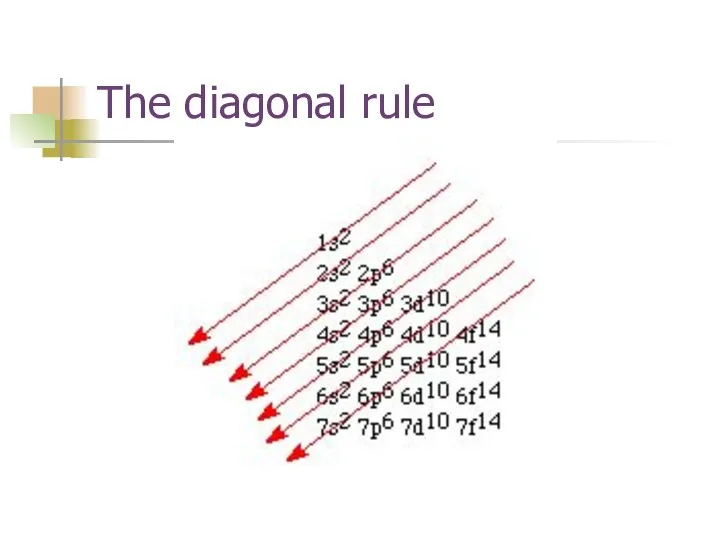
- 14. The diagonal rule
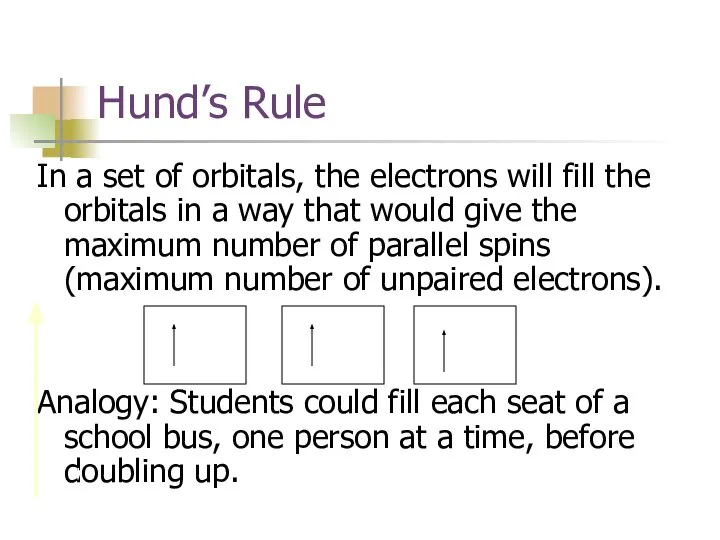
- 15. Hund’s Rule In a set of orbitals, the electrons will fill the orbitals in a way
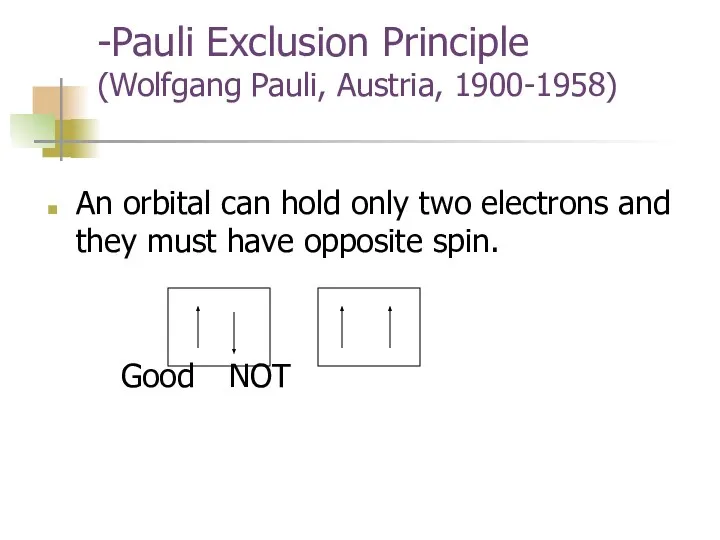
- 16. -Pauli Exclusion Principle (Wolfgang Pauli, Austria, 1900-1958) An orbital can hold only two electrons and they
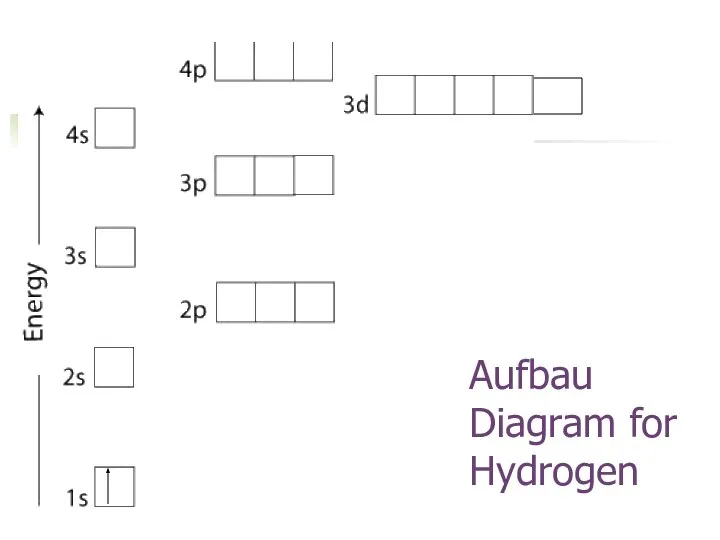
- 17. Aufbau Diagram for Hydrogen
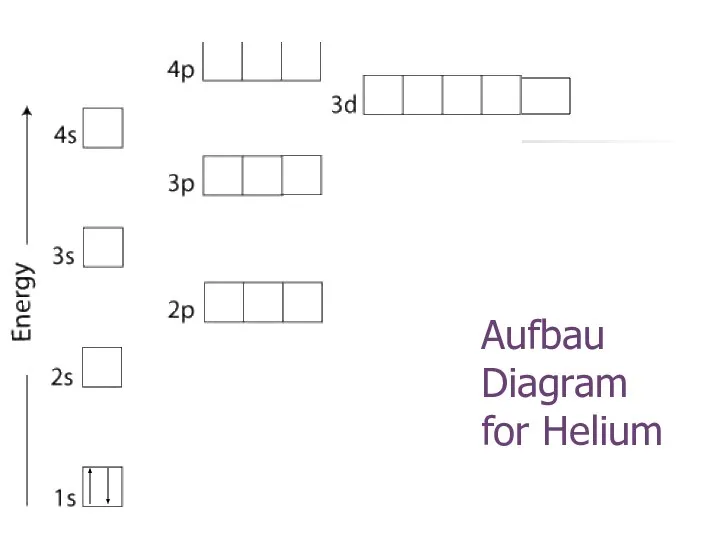
- 18. Aufbau Diagram for Helium
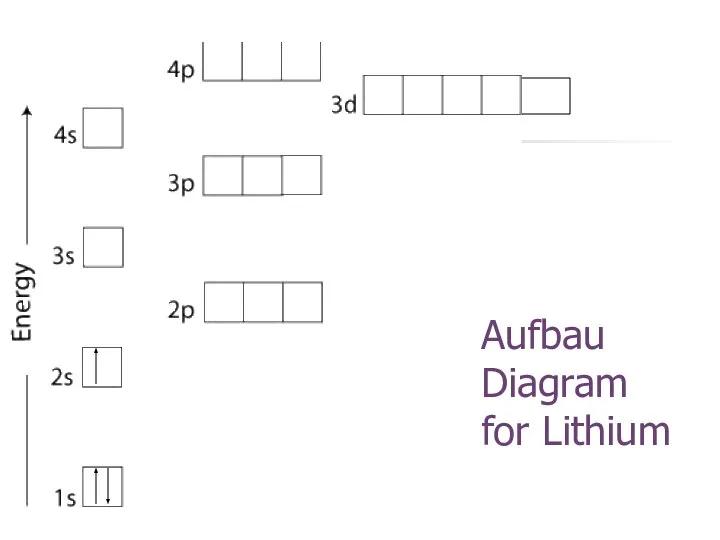
- 19. Aufbau Diagram for Lithium
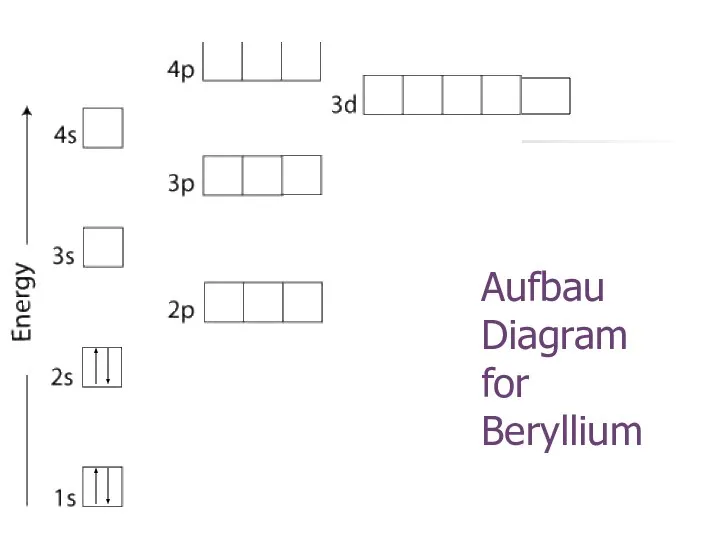
- 20. Aufbau Diagram for Beryllium
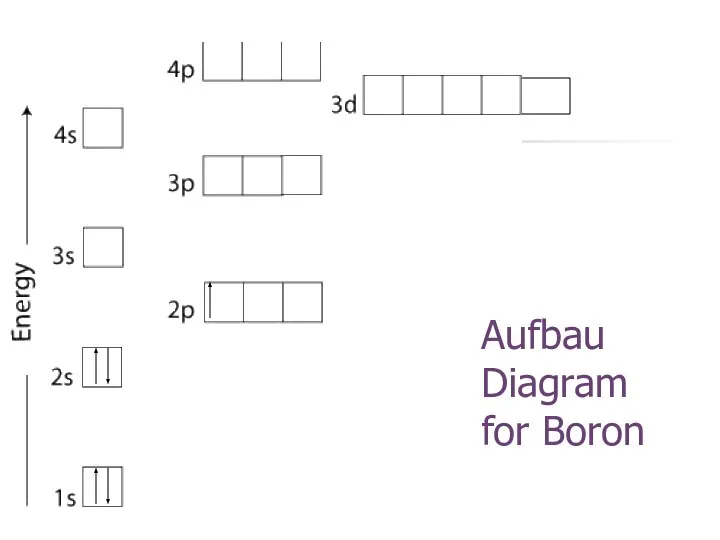
- 21. Aufbau Diagram for Boron
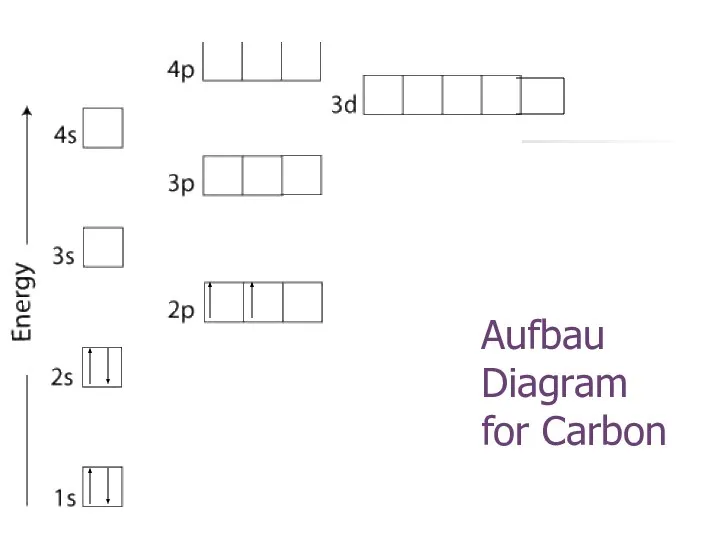
- 22. Aufbau Diagram for Carbon
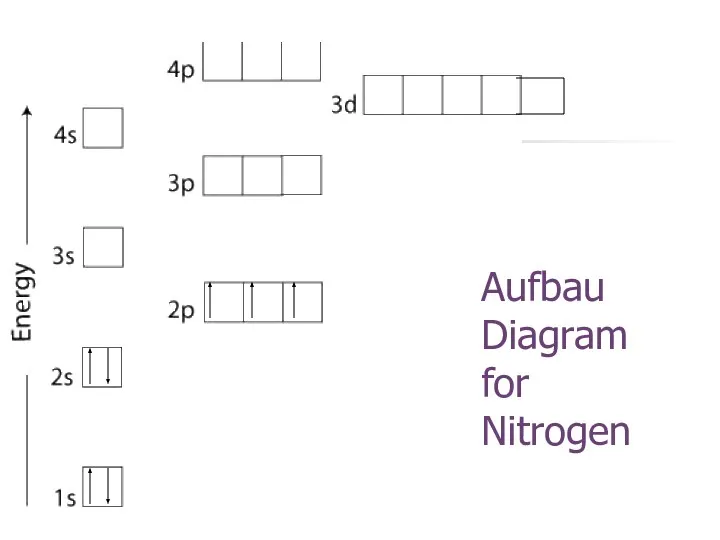
- 23. Aufbau Diagram for Nitrogen
- 24. Notations of Electron Configurations Standard Shorthand
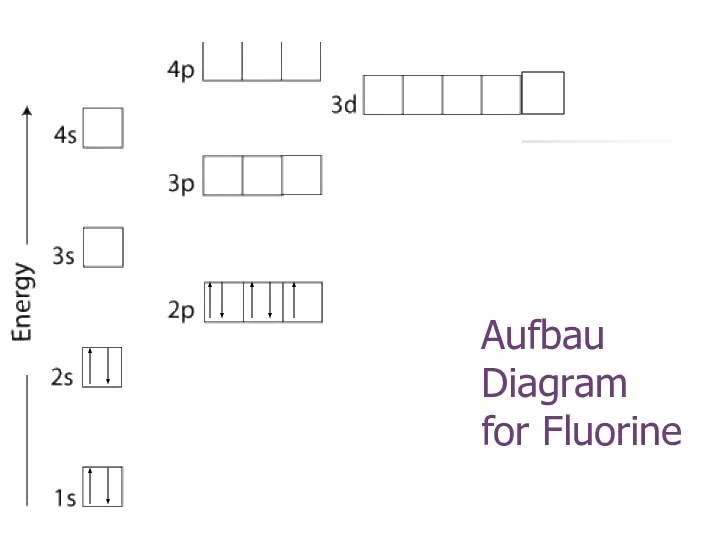
- 25. Aufbau Diagram for Fluorine
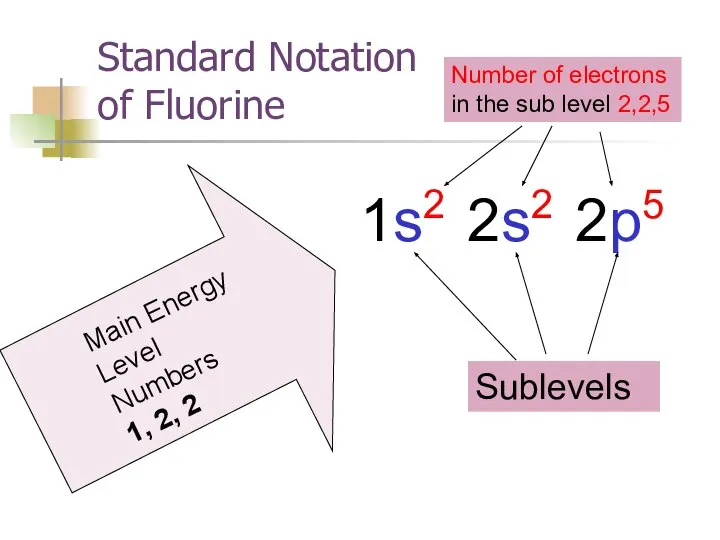
- 26. Standard Notation of Fluorine Main Energy Level Numbers 1, 2, 2 Sublevels Number of electrons in
- 27. Shorthand Notation Use the last noble gas that is located in the periodic table right before
- 29. Скачать презентацию




 Весёлая математика
Весёлая математика Асэнсаванне еўрапейскай культуры Новага часу асветнікамі Беларусі
Асэнсаванне еўрапейскай культуры Новага часу асветнікамі Беларусі ВЕТРЫ ЗЕМЛИ
ВЕТРЫ ЗЕМЛИ С днём пионерии
С днём пионерии Концепция рекламно-художественного оформления города
Концепция рекламно-художественного оформления города Михаил Пришвин. Школьная Робинзонада
Михаил Пришвин. Школьная Робинзонада Копия презентации на www.demos.su/integrat/secur.htm
Копия презентации на www.demos.su/integrat/secur.htm Презентация на тему Слово и слог 1 класс
Презентация на тему Слово и слог 1 класс Нужно помогать людям!!!!!!!!!!!!
Нужно помогать людям!!!!!!!!!!!! Арта. Юбилей
Арта. Юбилей Учебный проект
Учебный проект Вопросы от ученого кота
Вопросы от ученого кота Презентация на тему РЕНТГЕНОВСКИЕ ЛУЧИ
Презентация на тему РЕНТГЕНОВСКИЕ ЛУЧИ  Всероссийский конкурс
Всероссийский конкурс Компетентностная задача №12. Поддержим отечественного производителя
Компетентностная задача №12. Поддержим отечественного производителя Степени сравнения наречий
Степени сравнения наречий 11
11 Контроль и регулирование
Контроль и регулирование  День России
День России Министерство экономического развития Российской Федерации Российская академия народного хозяйства и государственной службы пр
Министерство экономического развития Российской Федерации Российская академия народного хозяйства и государственной службы пр Презентация на тему Насекомые
Презентация на тему Насекомые  Развитие представлений личности по Рубинштейну С.Л
Развитие представлений личности по Рубинштейну С.Л Паустовский «Жильцы старого дома» 3 класс
Паустовский «Жильцы старого дома» 3 класс Карвинг
Карвинг Презентация
Презентация Презентация на тему Биосфера как единая экосистема Земли
Презентация на тему Биосфера как единая экосистема Земли Кафедра таможенного дела Магистерская диссертация Эффективность таможенно-тарифной политики в создании безбарьерной внешнетор
Кафедра таможенного дела Магистерская диссертация Эффективность таможенно-тарифной политики в создании безбарьерной внешнетор