Слайд 3Make a list of inferences about any properties of objects in the

box.
How could you learn more about the objects in the box without opening the box?
Scientist face these same questions as they try to learn more about atoms.
Слайд 4Quantum Numbers
Quantum numbers specify the address of each electron in an

atom. There are four types of quantum numbers:
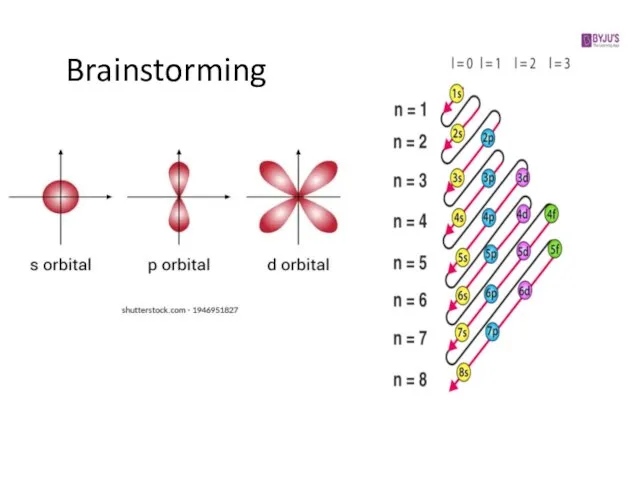
1. Principal quantum number, n → energy level (shell)
2. Secondary quantum number, l → subshell (s, p, d, f)
3. Magnetic quantum number, ml → orbital
4. Spin quantum number, ms → spin type of electro
There are no two electrons in an atom that can have the same four quantum numbers. Each electron has a unique address, like a family living in a flat. This is Pauli's Exclusion Principle.
Слайд 51. The principal quantum number, n
determines the size and energy of an

atom (larger n means bigger atoms and higher energy),
can take an integer value n = 1, 2, 3, 4 ... or (K, L, M, N...),
all electrons in an atom with the same value are said to belong to the same shell.
Слайд 62. Secondary quantum number, l
determines the overall shape of the orbital

within a shell
affects orbital energies (bigger l = higher energy)
all electrons in an atom with the same value of ‘l’ are said to belong to the same subshell
has integer values between 0 and n-1
may be called the “orbital angular momentum quantum number”
Слайд 73. Magnetic quantum number, ml
determines the orientation of orbitals within a subshell

does not affect orbital energy
has integer values between -I and +I
the number of ml values within a subshell is the number of orbitals within a subshell
s, p, d and f subshells includes 1, 3, 5 and 7 orbitals respectively.
Слайд 84. Spin quantum number, ms
each orbital may contain two electrons at most

several experimental observations can be explained by treating the electron as though it were spinning
spin affects the electron behave like a tiny magnet
spin can be clockwise (+1/2) or counterclockwise (-1/2)
Слайд 9Solving problems
Example 1
Find the values of quantum numbers for hydrogen atom.
Example
2
Show the values of possible quantum numbers for magnesium atom.( 12Mg)
Слайд 10Electron configuration
In 1925 Wolfgang Pauli stated his exclusion principle;
‘In the
same atom, two electrons may not have identical sets of all quantum numbers.’
According to this principle, the quantum numbers, n, l, ml, and ms, can never be identical for two electrons in an atom.
The Aufbau process
The Aufbau principle basically states that the lowest energy orbitals are filled first.
Hund’s rule states that;
the electrons are distributed among the orbitals of a subshell of the same energy in a way that gives the maximum number of unpaired electrons with parallel spin.
Слайд 111s2, 2s2, 2p6, 3s2, 3p6, 4s2, 3d10, 4p6, 5s2, 4d10, 5p6, 6s2,

4f14, 5d10, 6p6, 7s2, 5f14, 6d10, 7p6









 Исторический экскурс в советскую систему образования и воспитания подрастающего поколения
Исторический экскурс в советскую систему образования и воспитания подрастающего поколения Комитет ООН по правам человека
Комитет ООН по правам человека Теория управления. Лекция
Теория управления. Лекция Презентация на тему Нормы употребления числительных в речи
Презентация на тему Нормы употребления числительных в речи Определение стоимости объекта недвижимости на примере жилого дома
Определение стоимости объекта недвижимости на примере жилого дома Екатеринбургский музей ИЗО
Екатеринбургский музей ИЗО ЗДОРОВЬЕ ЧЕЛОВЕКА
ЗДОРОВЬЕ ЧЕЛОВЕКА «Точно в цель!» Первый благотворительный боулинг-турнир для корпоративных команд, посвященный Дню Защитника Отечества21 февраля
«Точно в цель!» Первый благотворительный боулинг-турнир для корпоративных команд, посвященный Дню Защитника Отечества21 февраля  Мировые деньги
Мировые деньги  Определение места для бивака и организация бивачных работ
Определение места для бивака и организация бивачных работ Не бывает дыма без огня
Не бывает дыма без огня Техника витража
Техника витража Управление общего и дошкольного образования Администрации города НорильскаНОВАЯ СИСТЕМА ОПЛАТЫ ТРУДА В ОБЩЕОБРАЗОВАТЕЛЬНЫХ УЧ
Управление общего и дошкольного образования Администрации города НорильскаНОВАЯ СИСТЕМА ОПЛАТЫ ТРУДА В ОБЩЕОБРАЗОВАТЕЛЬНЫХ УЧ Руснарбанк
Руснарбанк Японское искусство
Японское искусство столовая будущего нашими глазами
столовая будущего нашими глазами Государственная Третьяковская галерея
Государственная Третьяковская галерея Энергосбережение и энергоэффективность
Энергосбережение и энергоэффективность Алтимат Фризби в России
Алтимат Фризби в России Томск - 2011
Томск - 2011 Хостел (фотографии)
Хостел (фотографии) Презентация на тему Карты Проппа
Презентация на тему Карты Проппа Презентация на тему Причастие как часть речи
Презентация на тему Причастие как часть речи Внутрикорпоративный имидж и его влияние на приверженность сотрудников организации на примере ООО «Милко»
Внутрикорпоративный имидж и его влияние на приверженность сотрудников организации на примере ООО «Милко» Понятие и виды коллизионных норм
Понятие и виды коллизионных норм © 2010 Promodowww.promodo.ru
© 2010 Promodowww.promodo.ru  Оценка потенциала торговли углеродными квотами (взгляд экспертов компании) Начальник Департамента cтратегии и зарубежных проек
Оценка потенциала торговли углеродными квотами (взгляд экспертов компании) Начальник Департамента cтратегии и зарубежных проек Знакомство с кислотами
Знакомство с кислотами