Содержание
- 2. Elements Science has come along way since Aristotle’s theory of Air, Water, Fire, and Earth. Scientists
- 3. Elements The elements, alone or in combinations, make up our bodies, our world, our sun, and
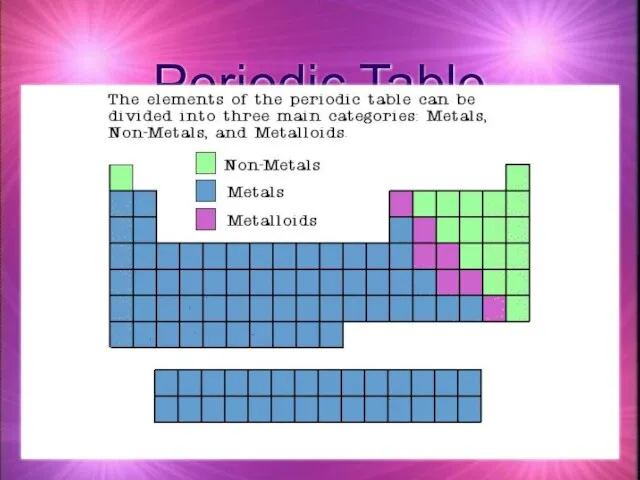
- 4. Periodic Table A great deal of information about an element can be gathered from its position
- 5. Periodic Table
- 6. Metals
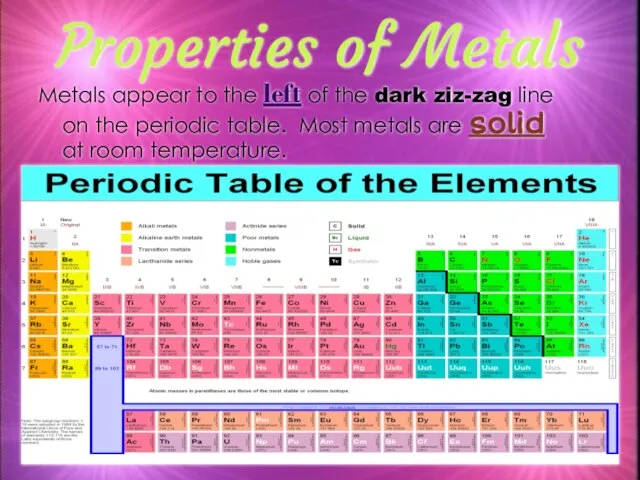
- 7. Properties of Metals Metals appear to the left of the dark ziz-zag line on the periodic
- 8. Properties of Metals Metals have luster. This means they are shiny
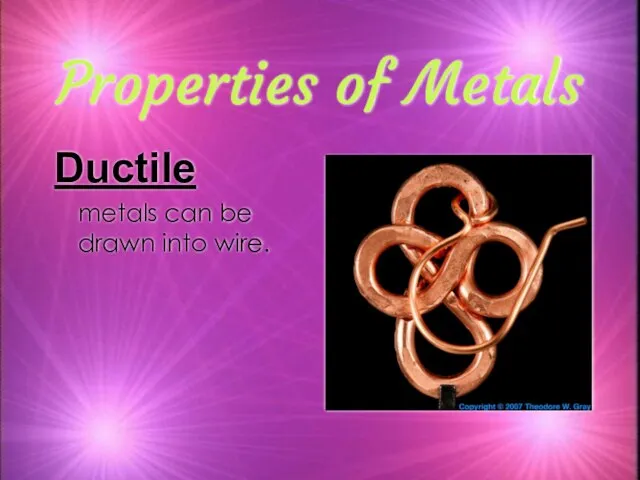
- 9. Properties of Metals Ductile metals can be drawn into wire.
- 10. Properties of Metals Malleable metals can be hammered into sheets
- 11. Properties of Metals Metals have a high melting point. They are also very dense.
- 12. Properties of Metals Conductors Metals are good conductors of electricity and heat
- 13. Properties of Metals A chemical property of metal is its reaction with water and oxygen. This
- 14. Metals Uses, Properties and Structures and Modifying Metals
- 15. Metals can be seen all around us We use metals because they have many useful properties
- 16. Metals (notes) Most elements are metals. 88 elements to the left of the stairstep line are
- 17. Exceptions to the rule Mercury is liquid at room temperature Alkali metals (group 1) can be
- 18. How would you test for hardness? Electrical conductivity? Heat conductivity?
- 19. Properties and Structures of Metals
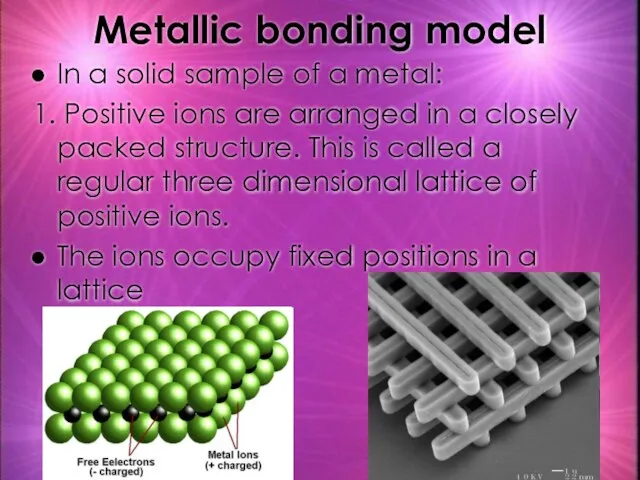
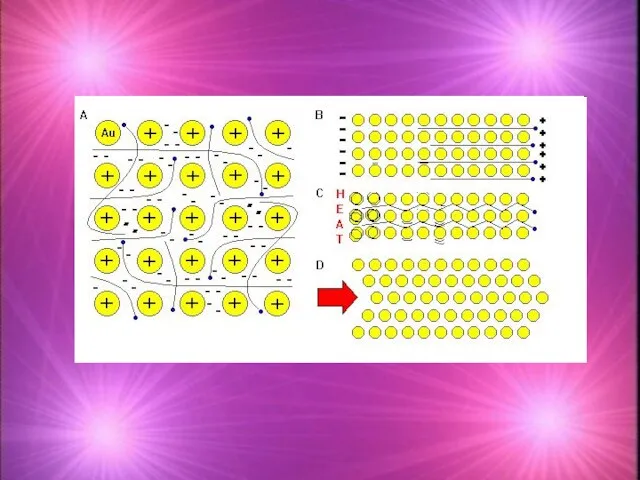
- 20. Metallic bonding model In a solid sample of a metal: 1. Positive ions are arranged in
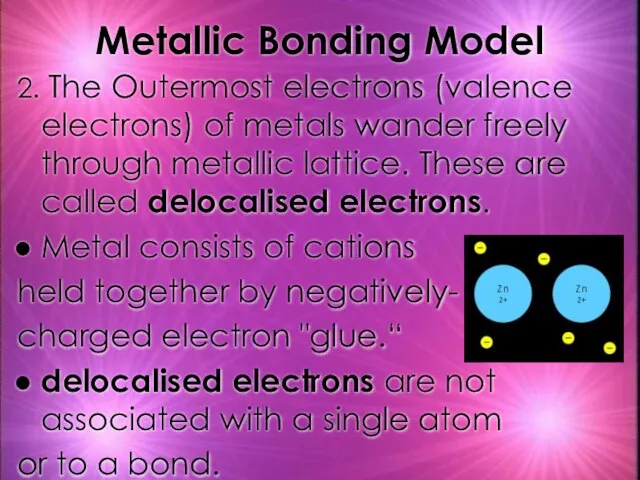
- 21. Metallic Bonding Model 2. The Outermost electrons (valence electrons) of metals wander freely through metallic lattice.
- 22. 3. The ions in the lattice are held together by electrostatic forces and delocalised electrons Electrostatic
- 23. Limitations to Metallic bonding model Some properties of metals cannot be explained by the metallic bonding
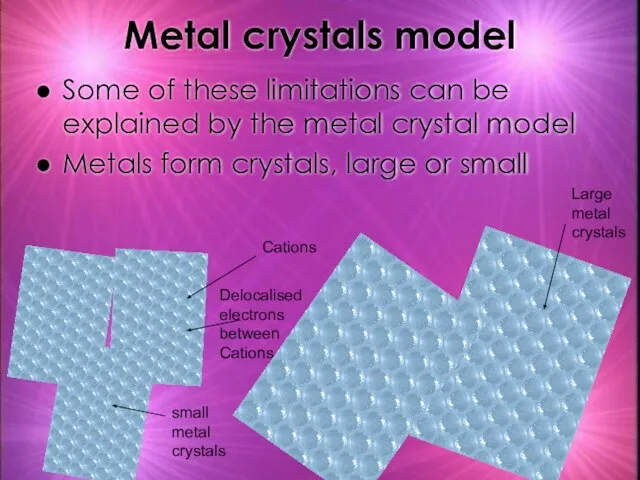
- 24. Metal crystals model Some of these limitations can be explained by the metal crystal model Metals
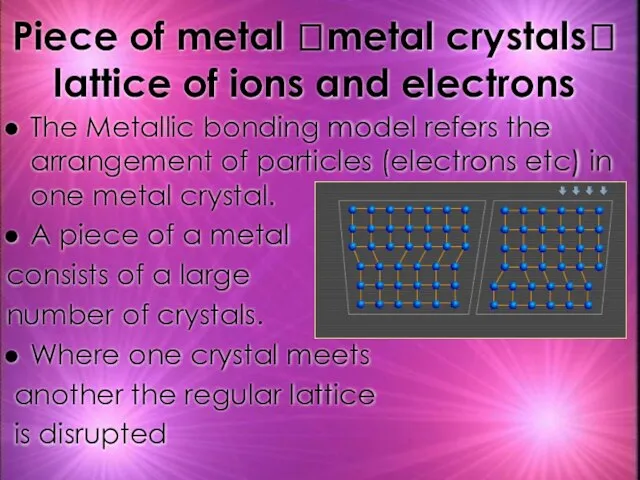
- 25. Piece of metal ?metal crystals? lattice of ions and electrons The Metallic bonding model refers the
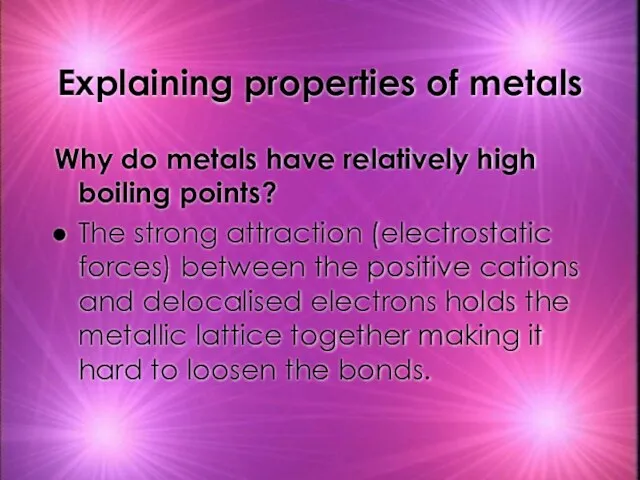
- 27. Explaining properties of metals Why do metals have relatively high boiling points? The strong attraction (electrostatic
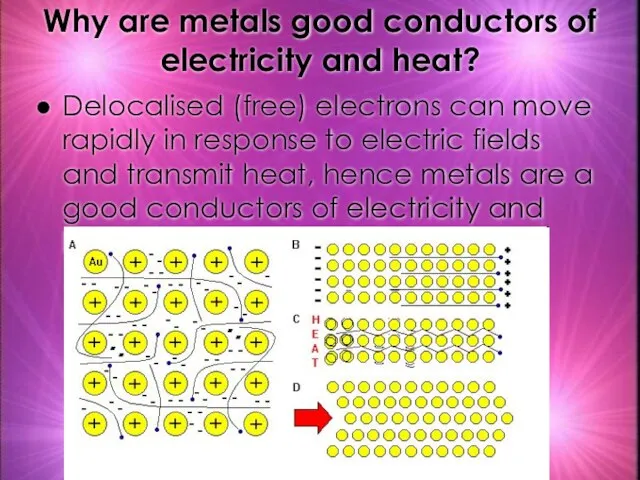
- 28. Why are metals good conductors of electricity and heat? Delocalised (free) electrons can move rapidly in
- 29. Why are metals malleable and ductile? The layers of atoms in metal are hard to pull
- 30. Why are metals hard? Strong electrostatic forces between the cations and electrons make it difficult to
- 31. Modifying metals Alloys, heat treatment and work hardening
- 32. Modifying Metals Few metals are used in their pure form Most metals need to be changed
- 33. A: Alloys Properties of metals can be significantly altered by adding other substances, usually a metal
- 34. White gold is an alloy of gold and at least one white metal, usually nickel or
- 35. 1. Substitutional alloys Substitutional alloys are made from elements that have similar chemical properties Example: Copper
- 36. 2. Interstitial alloys In interstitial alloys a small proportion of a smaller atoms is added to
- 37. B: Hardening Metals The way a metal is prepared can have a large impact on how
- 39. Скачать презентацию

























 и формирование отчетности
и формирование отчетности Презентация на тему Швейные товары
Презентация на тему Швейные товары  Филологи
Филологи АУДИТ
АУДИТ Проектирование СКС коттеджного участка из бруса
Проектирование СКС коттеджного участка из бруса Интеграция риск-менеджмента в ключевые процессы принятия решений
Интеграция риск-менеджмента в ключевые процессы принятия решений Проект: Юридическое сопровождение дополнительной эмиссии обыкновенных акций, размещаемых по открытой подписке
Проект: Юридическое сопровождение дополнительной эмиссии обыкновенных акций, размещаемых по открытой подписке Выполненное и планируемое благоустройство
Выполненное и планируемое благоустройство Вопросы составления и заверения отчетности получателей бюджетных средств в территориальных органах Федерального казначейства
Вопросы составления и заверения отчетности получателей бюджетных средств в территориальных органах Федерального казначейства Чудесные превращения воды в природе (3 класс)
Чудесные превращения воды в природе (3 класс) Волга – главный символ нашего края
Волга – главный символ нашего края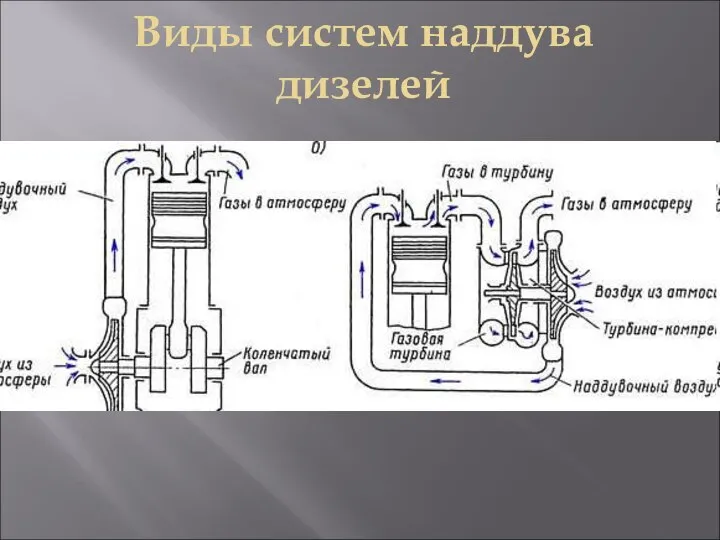 Виды систем наддува дизелей на судне. Аксиально-поршневой насос
Виды систем наддува дизелей на судне. Аксиально-поршневой насос Гражданское общество. Задания для выполнения
Гражданское общество. Задания для выполнения Природные и экологические опасности
Природные и экологические опасности London
London  Семейное право
Семейное право Morphonologie der Verben IPrasens
Morphonologie der Verben IPrasens Www.auditexpo.ru. Основные типы клиентов: Аналитический - ключевой вопрос: «Как?», «Зачем?» Рассудителен, Логичен, Собран, Нужны детали, Изуч
Www.auditexpo.ru. Основные типы клиентов: Аналитический - ключевой вопрос: «Как?», «Зачем?» Рассудителен, Логичен, Собран, Нужны детали, Изуч Мотивация продавцов-промоутеров SEB
Мотивация продавцов-промоутеров SEB Выражение характера человека в изображении. Женский образ русских сказок
Выражение характера человека в изображении. Женский образ русских сказок Портфолио
Портфолио Школьный музей "Родные истоки"
Школьный музей "Родные истоки" Тип, имя и значение переменной
Тип, имя и значение переменной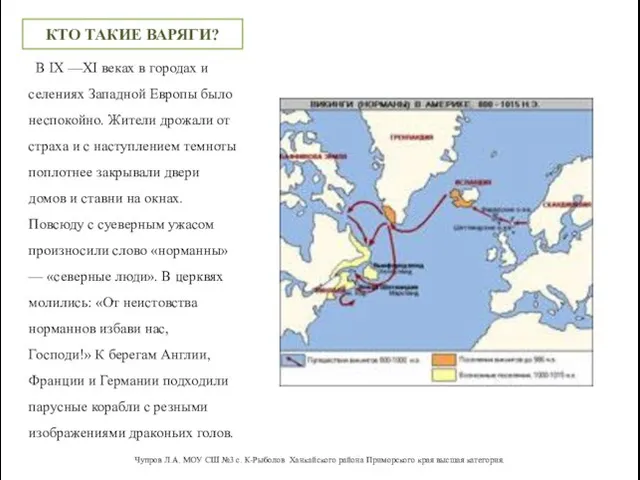 Путь из Варяг в Греки
Путь из Варяг в Греки Масштабируемое решениеот ЛПУ до региона
Масштабируемое решениеот ЛПУ до региона Агроэкосистеемы, или аграрные экологические системы
Агроэкосистеемы, или аграрные экологические системы Религия как одна из форм культуры
Религия как одна из форм культуры Угольная кислота
Угольная кислота