Содержание
- 2. ATOMIC STRUCTURE INTRODUCTION This Powerpoint show is one of several produced to help students understand selected
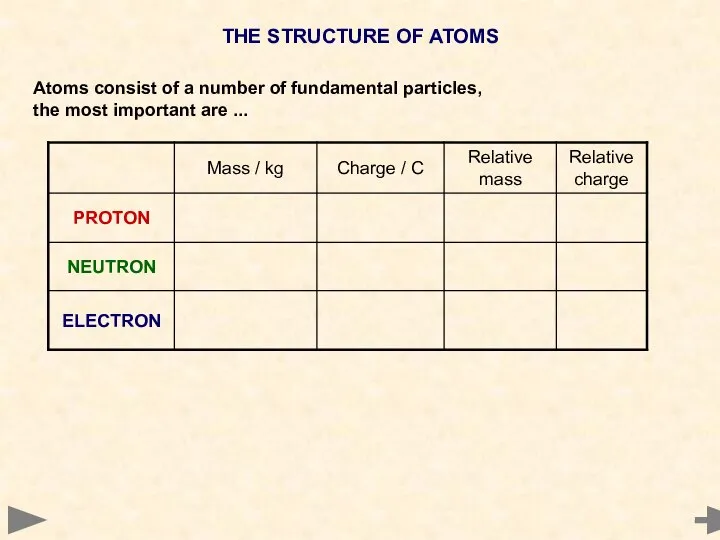
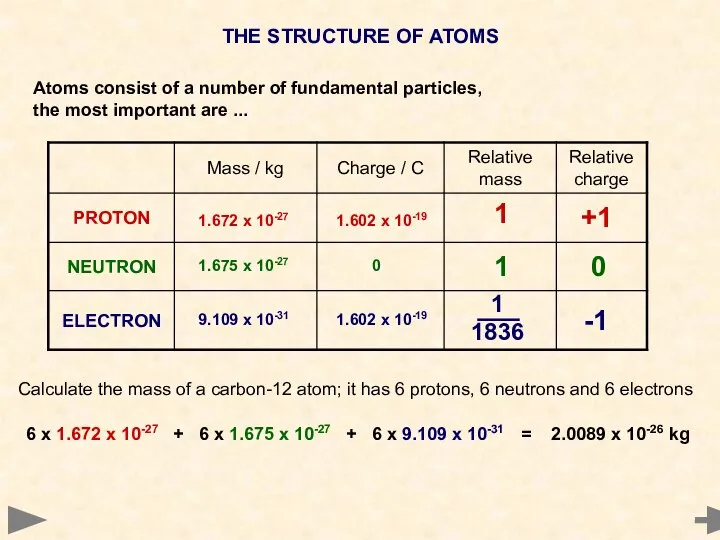
- 3. THE STRUCTURE OF ATOMS Atoms consist of a number of fundamental particles, the most important are
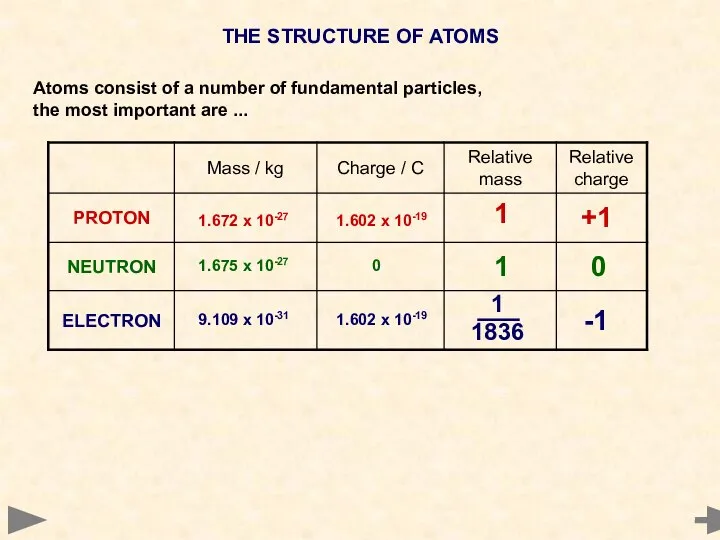
- 4. THE STRUCTURE OF ATOMS Atoms consist of a number of fundamental particles, the most important are
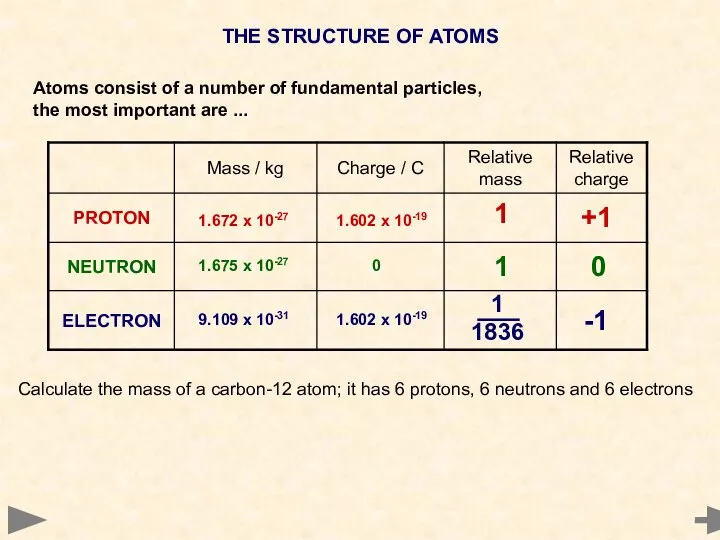
- 5. THE STRUCTURE OF ATOMS Atoms consist of a number of fundamental particles, the most important are
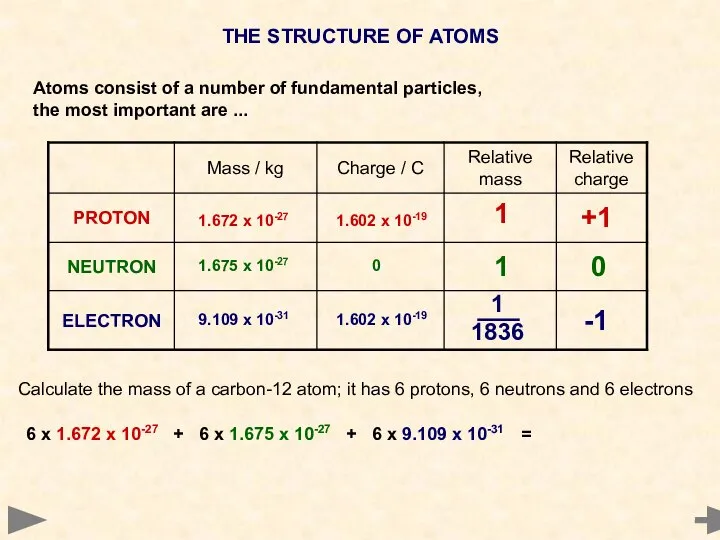
- 6. THE STRUCTURE OF ATOMS Atoms consist of a number of fundamental particles, the most important are
- 7. THE STRUCTURE OF ATOMS Atoms consist of a number of fundamental particles, the most important are
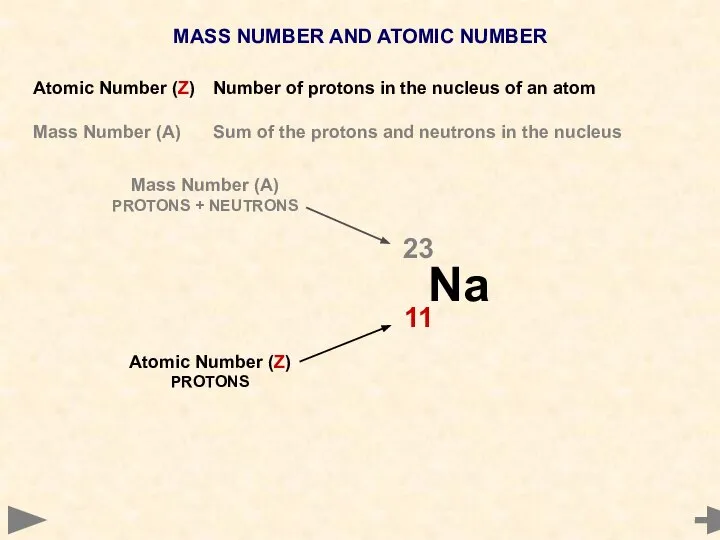
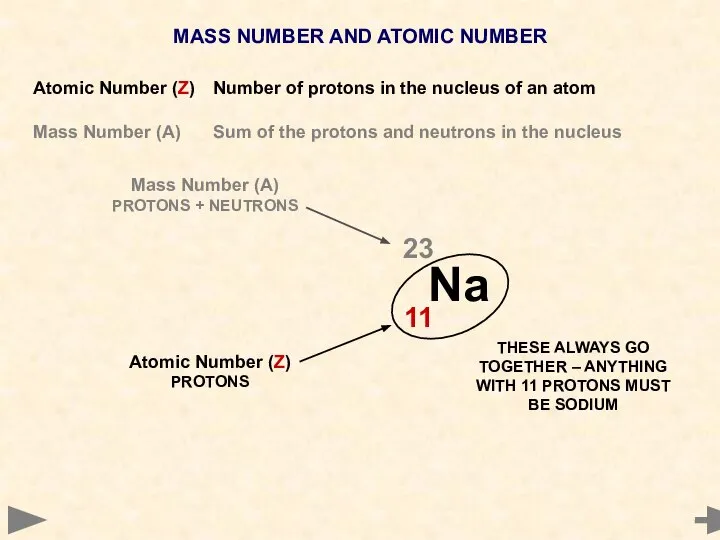
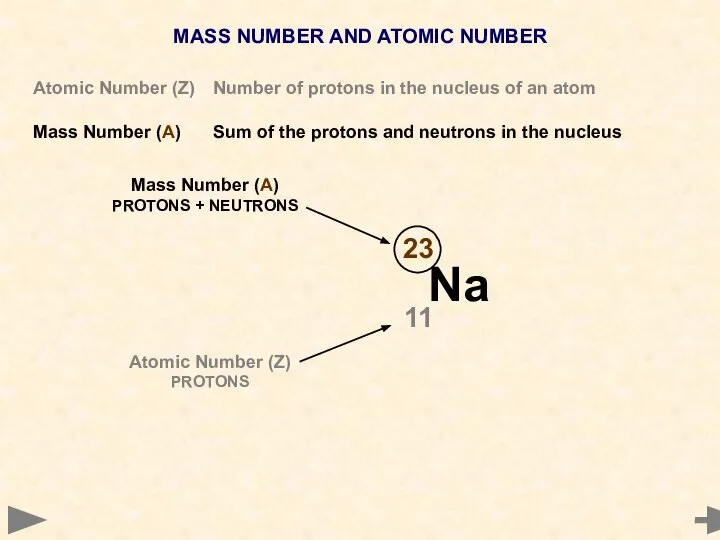
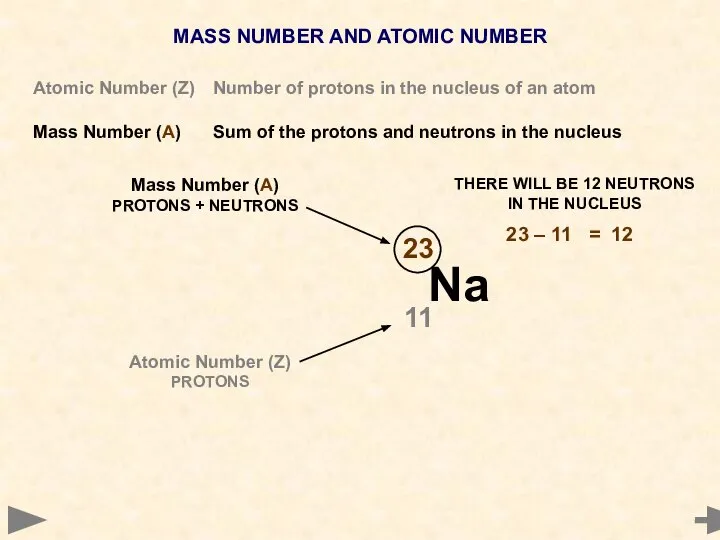
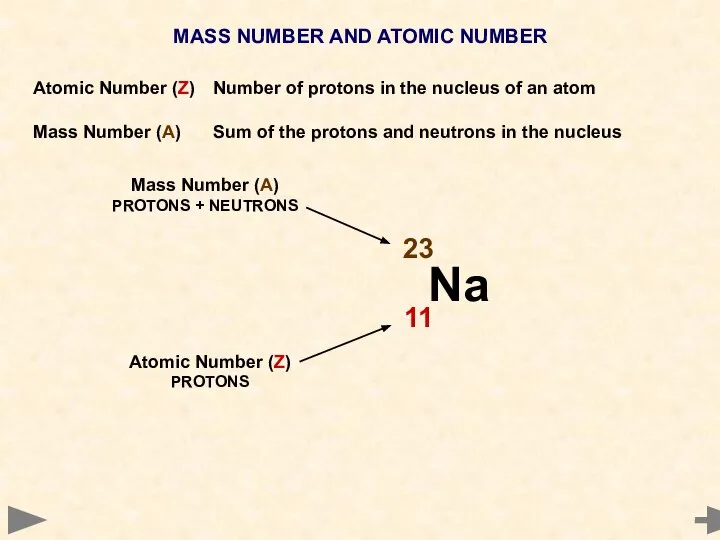
- 8. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
- 9. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
- 10. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
- 11. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
- 12. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
- 13. MASS NUMBER AND ATOMIC NUMBER Atomic Number (Z) Number of protons in the nucleus of an
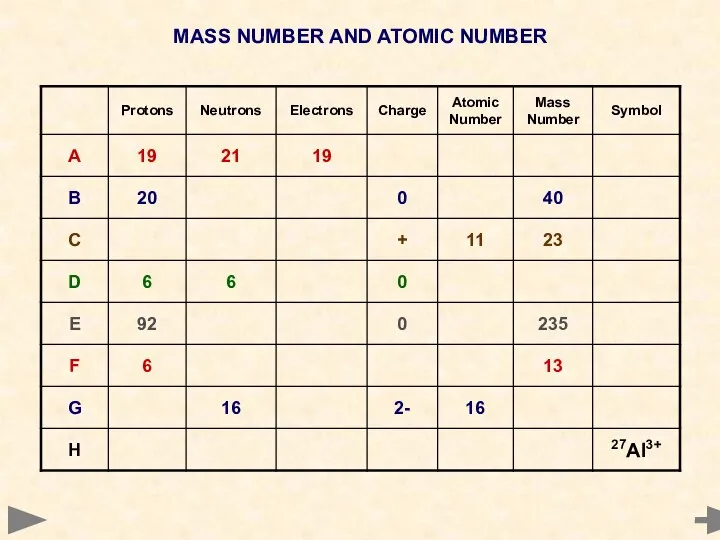
- 14. MASS NUMBER AND ATOMIC NUMBER
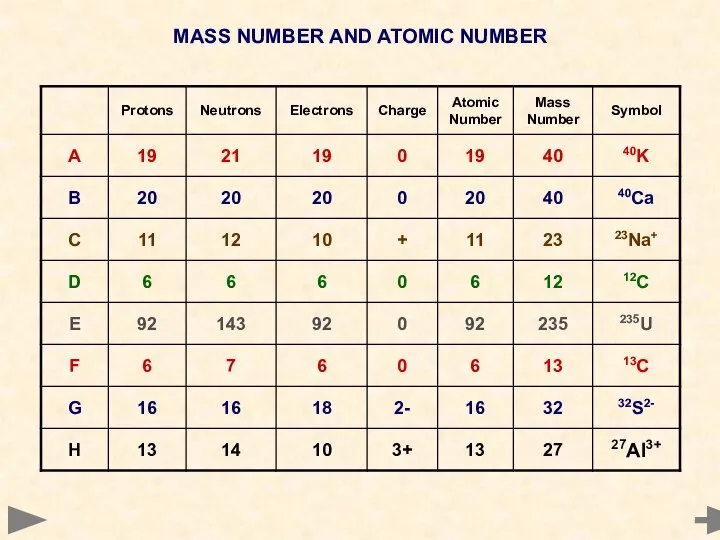
- 15. MASS NUMBER AND ATOMIC NUMBER
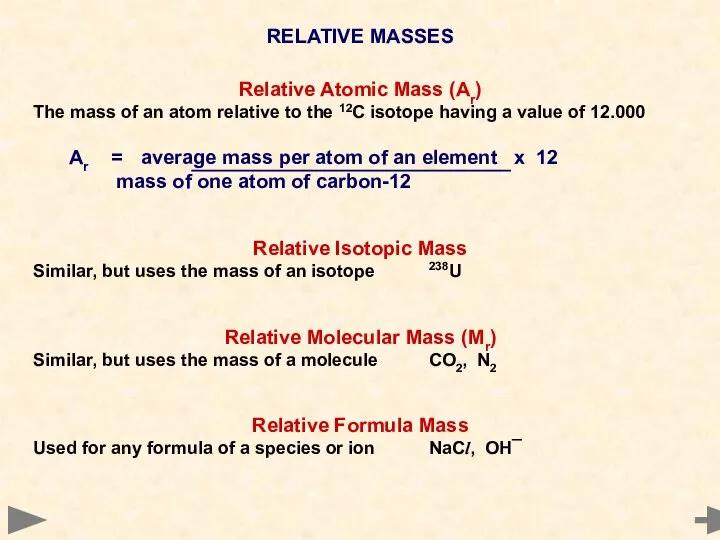
- 16. RELATIVE MASSES Relative Atomic Mass (Ar) The mass of an atom relative to the 12C isotope
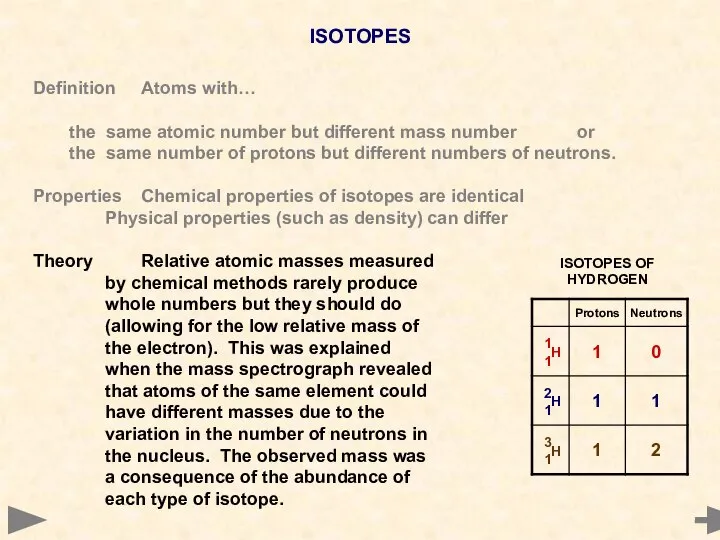
- 17. ISOTOPES Definition Atoms with… the same atomic number but different mass number or the same number
- 18. ISOTOPES Definition Atoms with… the same atomic number but different mass number or the same number
- 19. ISOTOPES Definition Atoms with… the same atomic number but different mass number or the same number
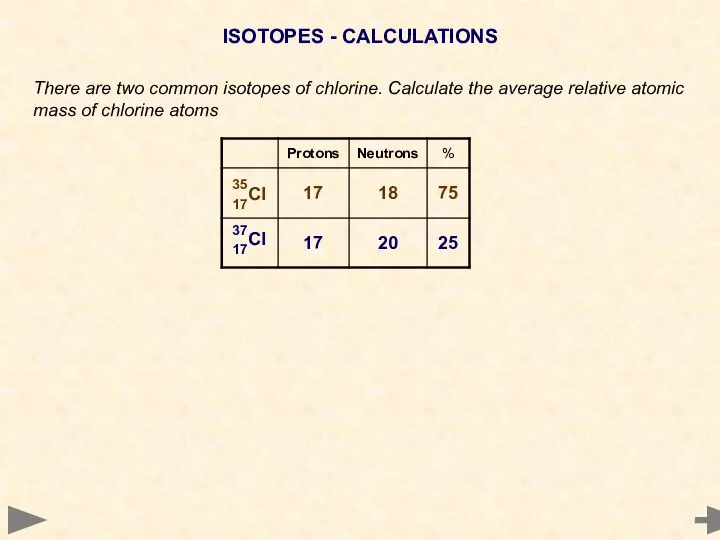
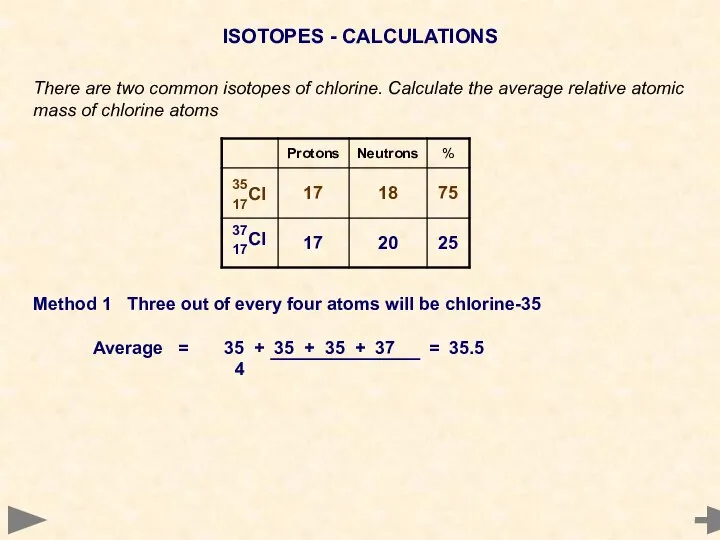
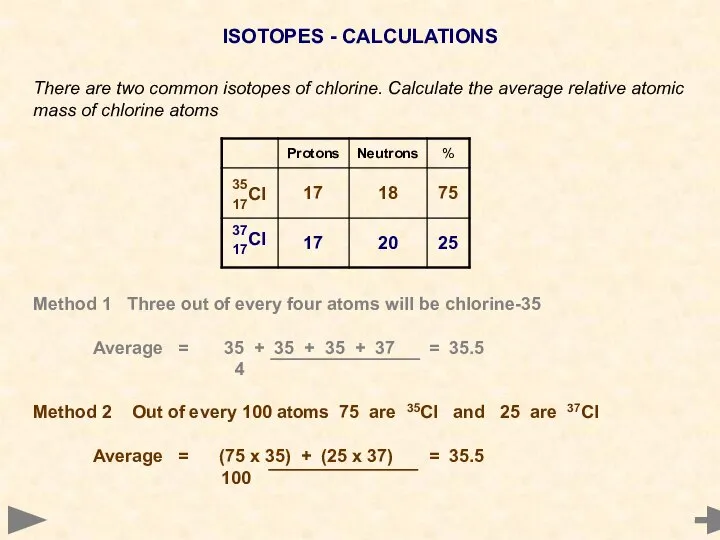
- 20. ISOTOPES - CALCULATIONS There are two common isotopes of chlorine. Calculate the average relative atomic mass
- 21. ISOTOPES - CALCULATIONS There are two common isotopes of chlorine. Calculate the average relative atomic mass
- 22. ISOTOPES - CALCULATIONS There are two common isotopes of chlorine. Calculate the average relative atomic mass
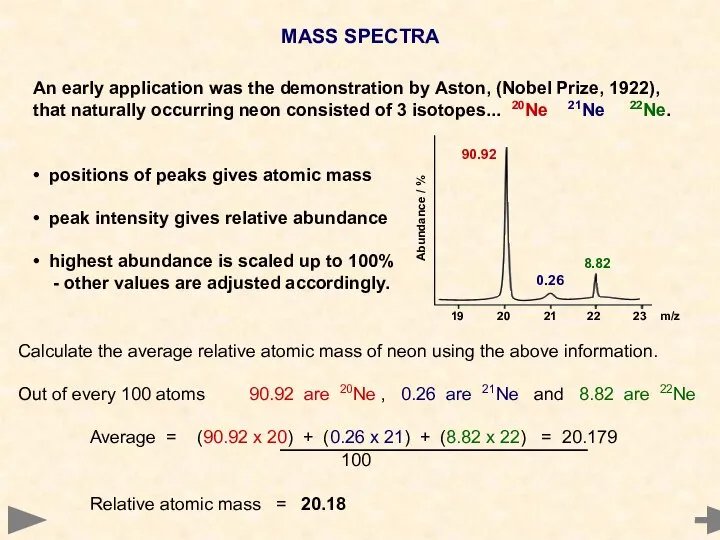
- 23. MASS SPECTRA An early application was the demonstration by Aston, (Nobel Prize, 1922), that naturally occurring
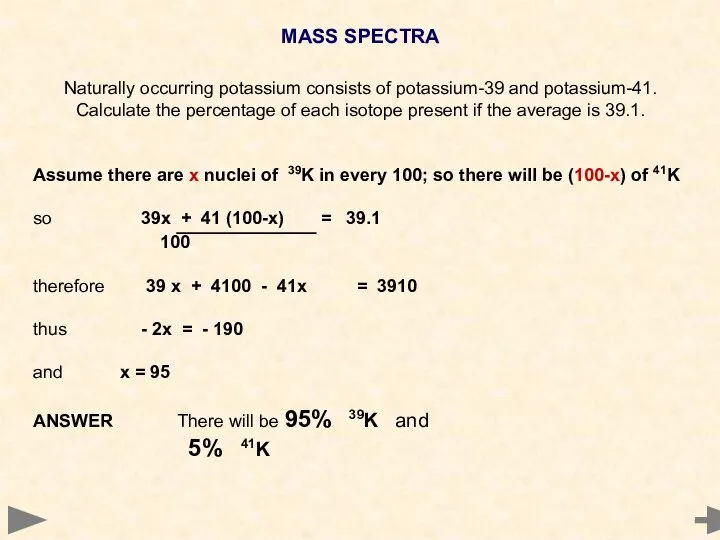
- 24. MASS SPECTRA Naturally occurring potassium consists of potassium-39 and potassium-41. Calculate the percentage of each isotope
- 26. Скачать презентацию




 Окислительно- восстановительные реакции в органической химии. Подготовка к ЕГЭ
Окислительно- восстановительные реакции в органической химии. Подготовка к ЕГЭ Углеводы. Общее понятие углеводов
Углеводы. Общее понятие углеводов Очистка хозяйственных и сточных вод от азота аммонийного с помощью нитрифицирующих бактерий
Очистка хозяйственных и сточных вод от азота аммонийного с помощью нитрифицирующих бактерий Распределение полициклических ароматических углеводородов в углях Кузнецкого угольного бассейна
Распределение полициклических ароматических углеводородов в углях Кузнецкого угольного бассейна 1
1 Химия 9 класс. Введение - повторение
Химия 9 класс. Введение - повторение Изучение основных породообразующих минералов горных пород. Лабораторная работа №1
Изучение основных породообразующих минералов горных пород. Лабораторная работа №1 Периодический закон и строение атома
Периодический закон и строение атома Лекция 6 Нарушения кислотнощелочного равновесия ацидозы, алкалозы
Лекция 6 Нарушения кислотнощелочного равновесия ацидозы, алкалозы Биогеотехнология
Биогеотехнология Презентация на тему Теория строения органических соединений
Презентация на тему Теория строения органических соединений  Природный газ
Природный газ Презентация на тему Основания
Презентация на тему Основания  Устойчивое развитие
Устойчивое развитие Сырье, продукты, параметры гидроочистки
Сырье, продукты, параметры гидроочистки Простые и сложные вещества
Простые и сложные вещества Гликоген. Синтез гликогена
Гликоген. Синтез гликогена Единые смеси
Единые смеси Интерактивная интеллектуальная игра. Юный химик
Интерактивная интеллектуальная игра. Юный химик Химическая связь
Химическая связь Простые и сложные вещества
Простые и сложные вещества Жиры – биологически важные органические соединения
Жиры – биологически важные органические соединения Ковалентная связь. Радикалы
Ковалентная связь. Радикалы Структурный тип
Структурный тип Натрий, свойства атома, химические и физические свойства
Натрий, свойства атома, химические и физические свойства Характеристика щелочных металлов (9 класс)
Характеристика щелочных металлов (9 класс) Характеристика кислорода и серы
Характеристика кислорода и серы Посвящение в химики
Посвящение в химики