Слайд 4VACCINE
Twenty vaccines are authorized by at least one national regulatory authority for public use:

one DNA vaccine (ZyCoV-D) two RNA vaccines (Pfizer–BioNTech and Moderna), ten conventional inactivated vaccines (BBIBP-CorV, Chinese Academy of Medical Sciences, CoronaVac, Covaxin, CoviVac, COVIran Barekat, FAKHRAVAC, Minhai-Kangtai, QazVac, and WIBP-CorV), five viral vector vaccines (Sputnik Light, Sputnik V, Oxford–AstraZeneca, Convidecia, and Janssen), and six subunit vaccines (Abdala, COVAX-19, EpiVacCorona, MVC-COV1901, Soberana 02, and ZF2001).[32][33] As of July 2021, 330 vaccine candidates were in various stages of development, with 102 in clinical research, including 30 in Phase I trials, 30 in Phase I–II trials, 25 in Phase III trials, and 8 in Phase IV development.[32]






 Современные компьютерные технологии в косметологии
Современные компьютерные технологии в косметологии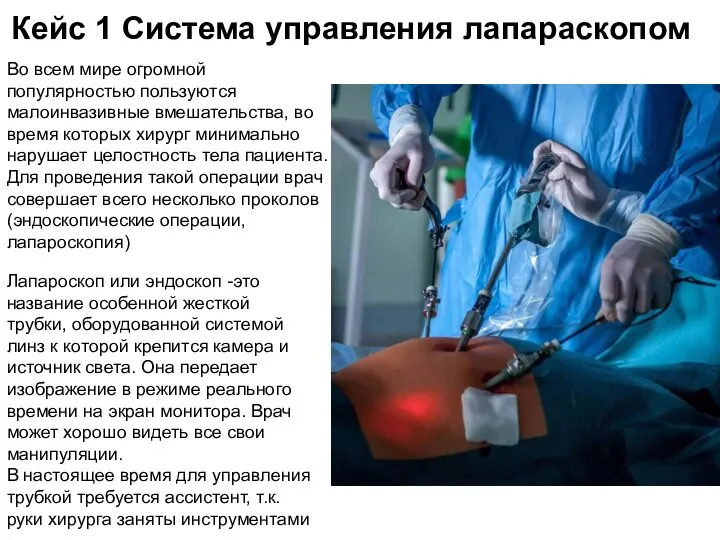 Система управления лапараскопом
Система управления лапараскопом Anatomy and Puncture Site
Anatomy and Puncture Site Швы кожи
Швы кожи Гигиена ухода за животными. Санитарно-гигиеническое значение ухода за кожей
Гигиена ухода за животными. Санитарно-гигиеническое значение ухода за кожей Помощь пациенту в осуществлении личной гигиены
Помощь пациенту в осуществлении личной гигиены Аллергия как измененная форма иммунного ответа
Аллергия как измененная форма иммунного ответа Осложнение беременности гестоз
Осложнение беременности гестоз Алгоритм дифференциальной диагностики инфаркта миокарда и расслаивающейся аневризмы
Алгоритм дифференциальной диагностики инфаркта миокарда и расслаивающейся аневризмы Artificial synthesis of insulin
Artificial synthesis of insulin Виды кровотечений
Виды кровотечений Проект #стопСПИД/ВИЧ
Проект #стопСПИД/ВИЧ Массаж , виды массажа и влияние массажа на функциональное состояние организма
Массаж , виды массажа и влияние массажа на функциональное состояние организма Холера
Холера Правильное питание
Правильное питание Аллергические заболевания и их профилактика
Аллергические заболевания и их профилактика Значение генетики в медицине
Значение генетики в медицине Гипобиотические и абиотические патологические процессы
Гипобиотические и абиотические патологические процессы Спинальный шок
Спинальный шок История развития машин скорой помощи в г. Тула
История развития машин скорой помощи в г. Тула Функциональная анатомия лимфатической системы
Функциональная анатомия лимфатической системы Полиомиелит
Полиомиелит Реактивный артрит при туберкулезе
Реактивный артрит при туберкулезе Транспортные положения
Транспортные положения Дифференциальная диагностика мочевого синдрома
Дифференциальная диагностика мочевого синдрома Improving the Odds Against Stroke
Improving the Odds Against Stroke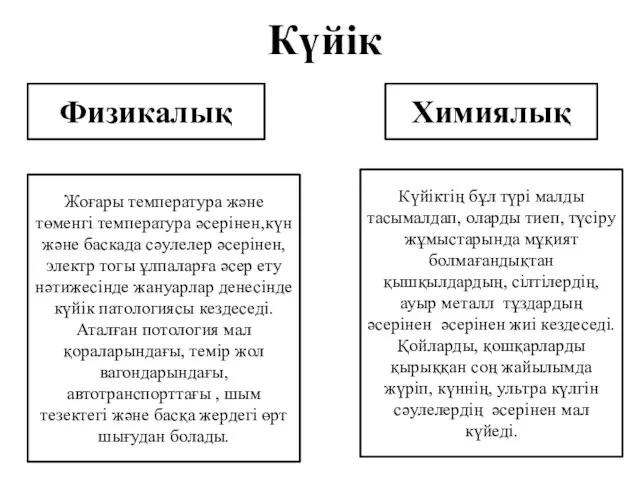 Күйік
Күйік Умственная отсталость, деменция
Умственная отсталость, деменция