Содержание
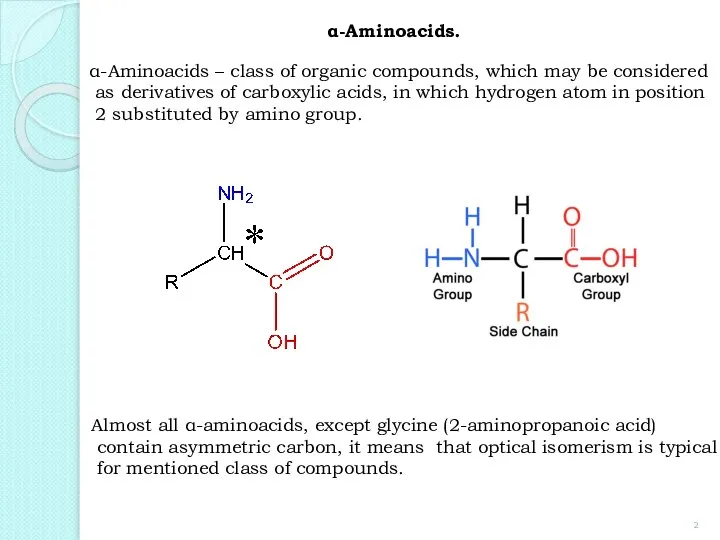
- 2. α-Aminoacids. α-Aminoacids – class of organic compounds, which may be considered as derivatives of carboxylic acids,
- 3. Classification By the one to which the carbon atom is attached an amino- (or imino-) group,
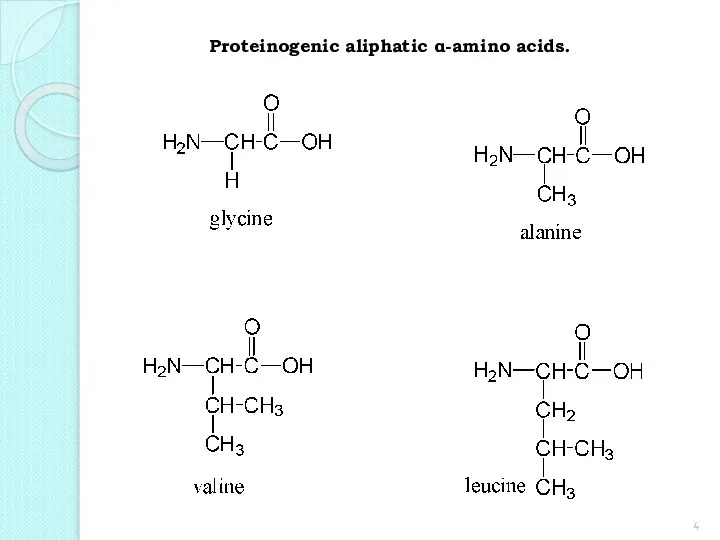
- 4. Proteinogenic aliphatic α-amino acids.
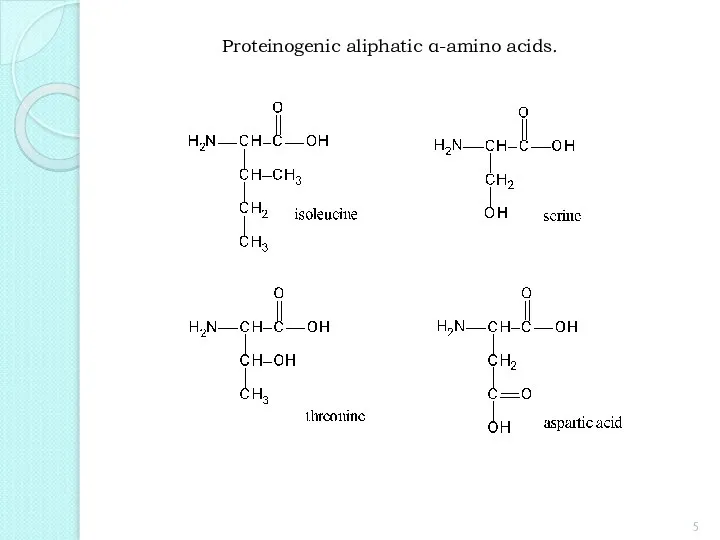
- 5. Proteinogenic aliphatic α-amino acids.
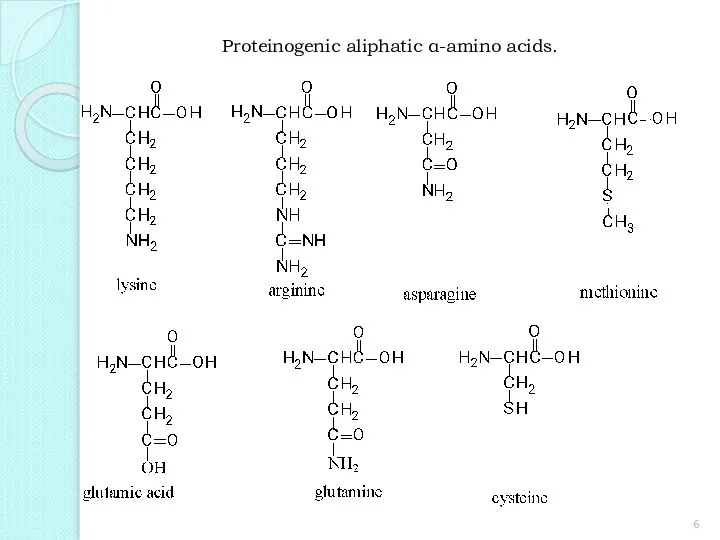
- 6. Proteinogenic aliphatic α-amino acids.
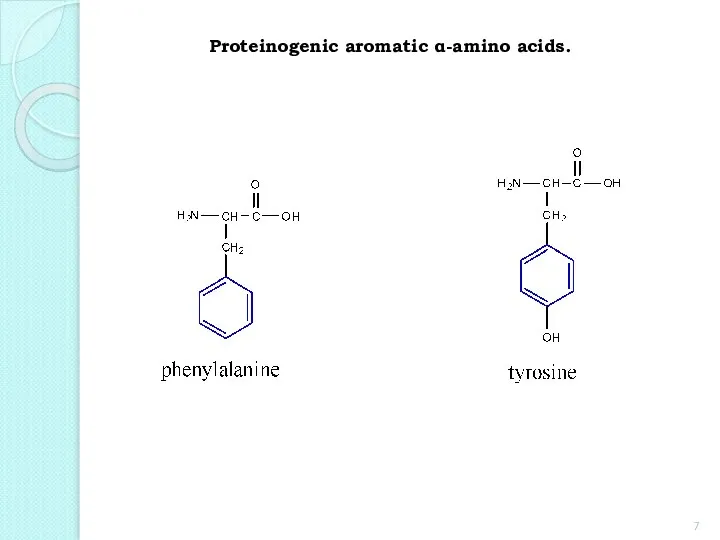
- 7. Proteinogenic aromatic α-amino acids.
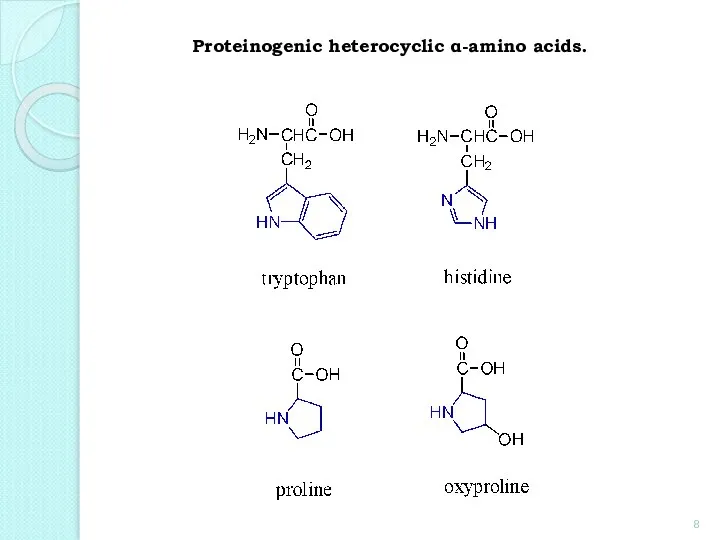
- 8. Proteinogenic heterocyclic α-amino acids.
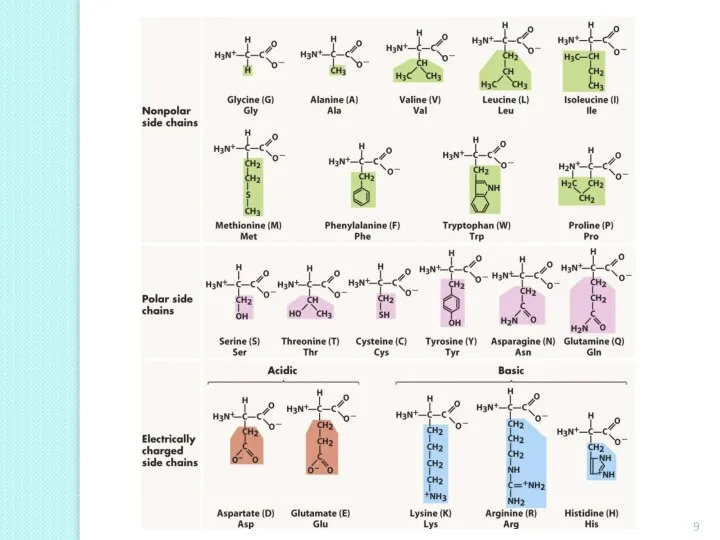
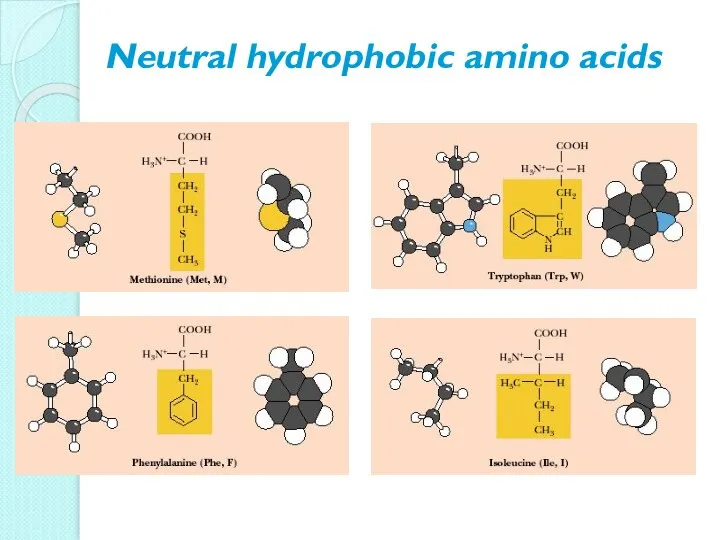
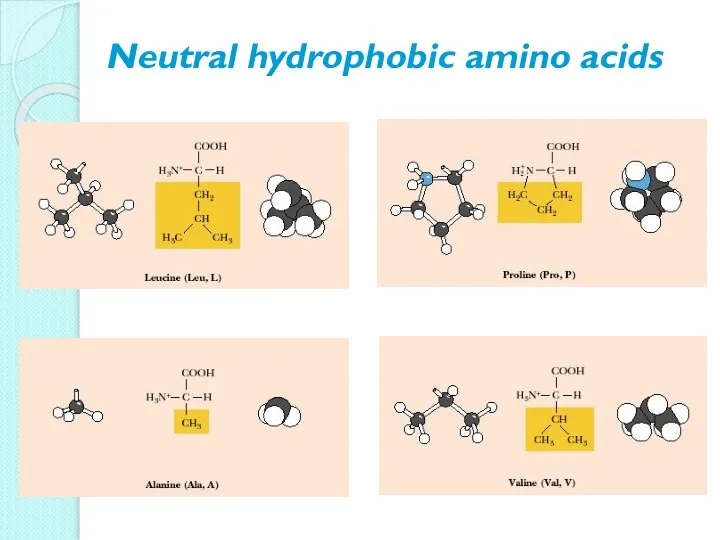
- 10. Neutral hydrophobic amino acids
- 11. Neutral hydrophobic amino acids
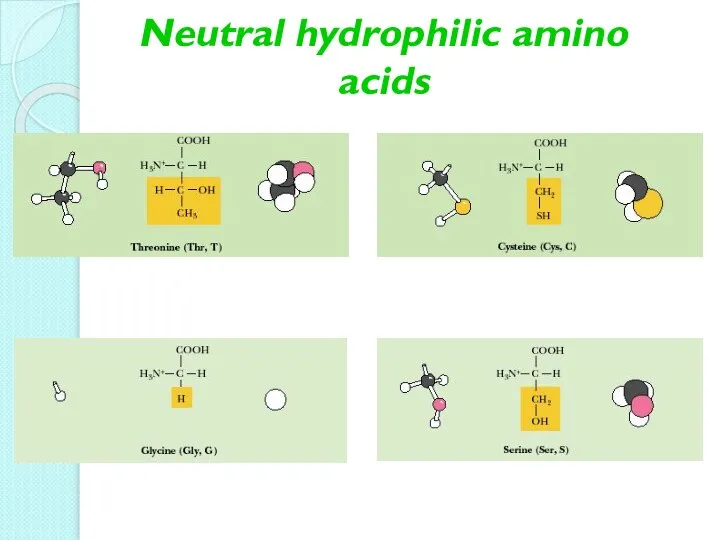
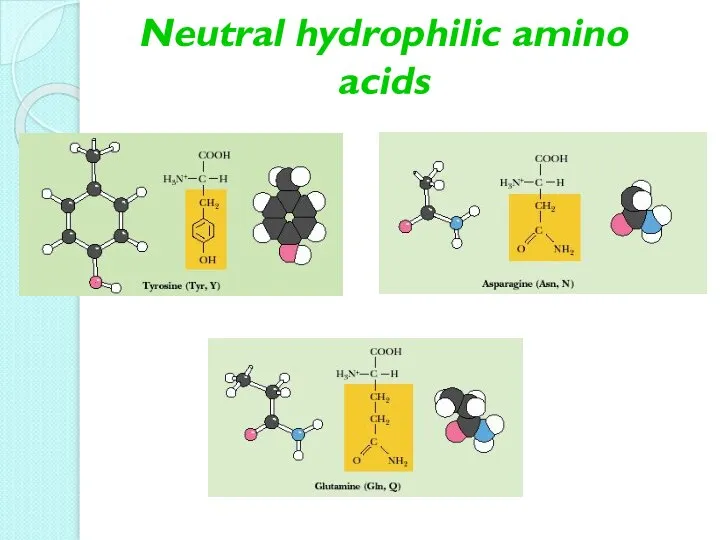
- 12. Neutral hydrophilic amino acids
- 13. Neutral hydrophilic amino acids
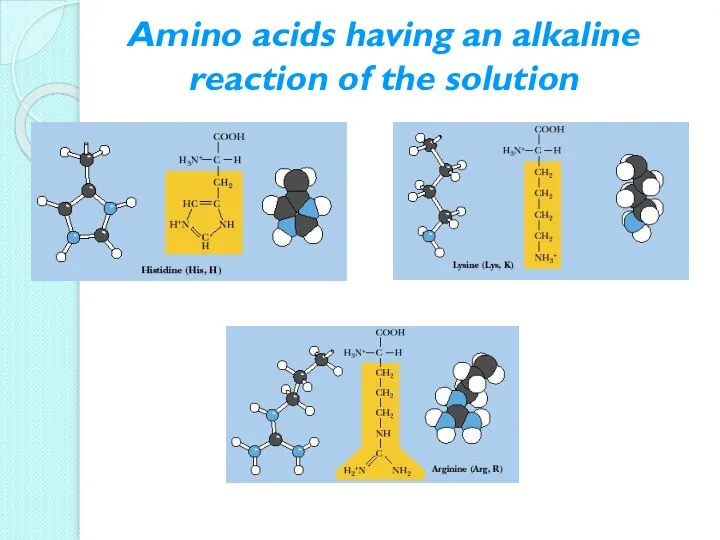
- 14. Amino acids having an alkaline reaction of the solution
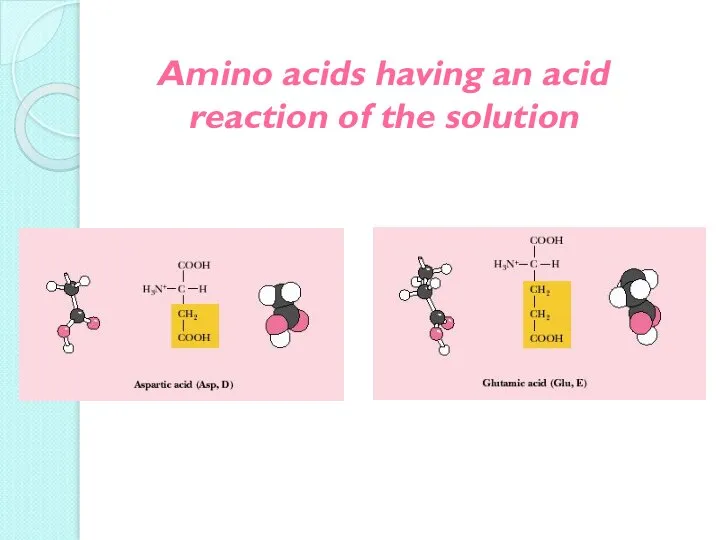
- 15. Amino acids having an acid reaction of the solution
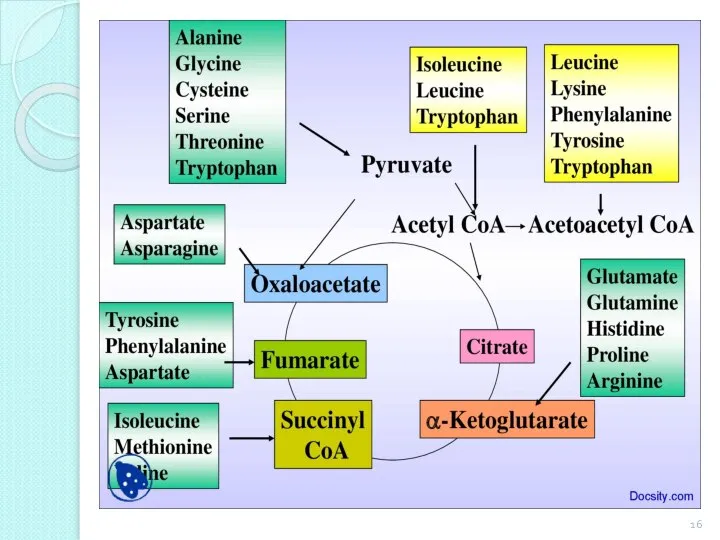
- 17. Non-essential AA: alanine, aspartic acid, asparagine, glutamic acid, glutamine, proline, glycine, serine. Enzyme systems of the
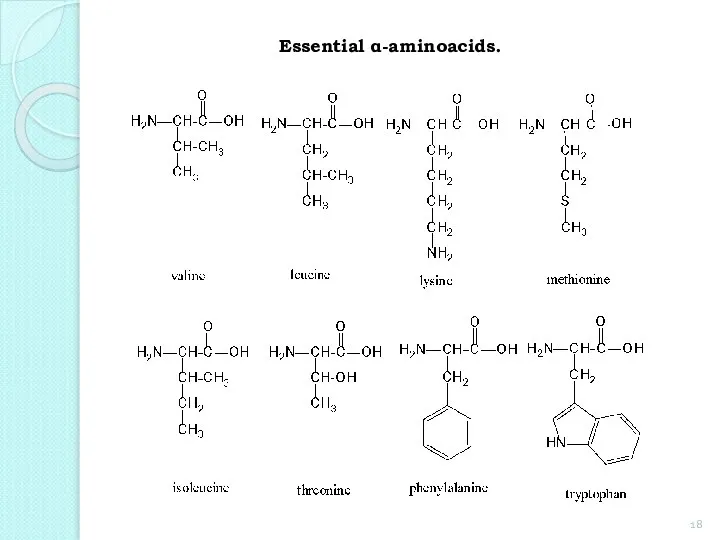
- 18. Essential α-aminoacids.
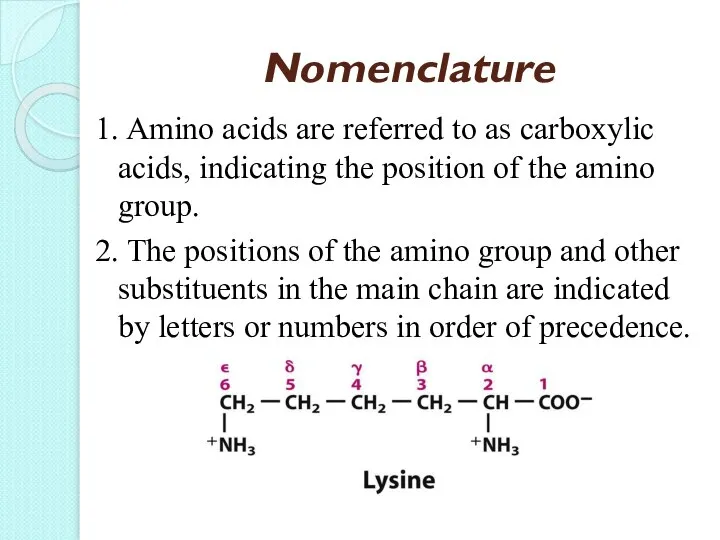
- 19. Nomenclature 1. Amino acids are referred to as carboxylic acids, indicating the position of the amino
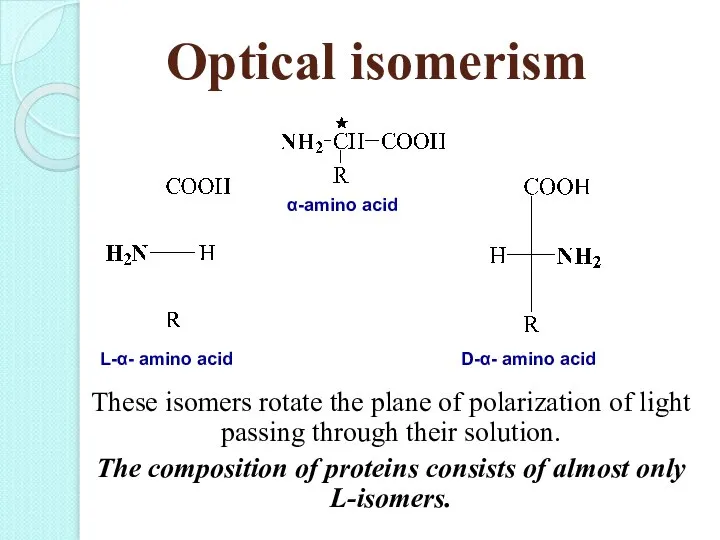
- 20. Optical isomerism These isomers rotate the plane of polarization of light passing through their solution. The
- 21. Physical properties Amino acids - colorless crystalline substances with high melting temperatures. Melting is accompanied by
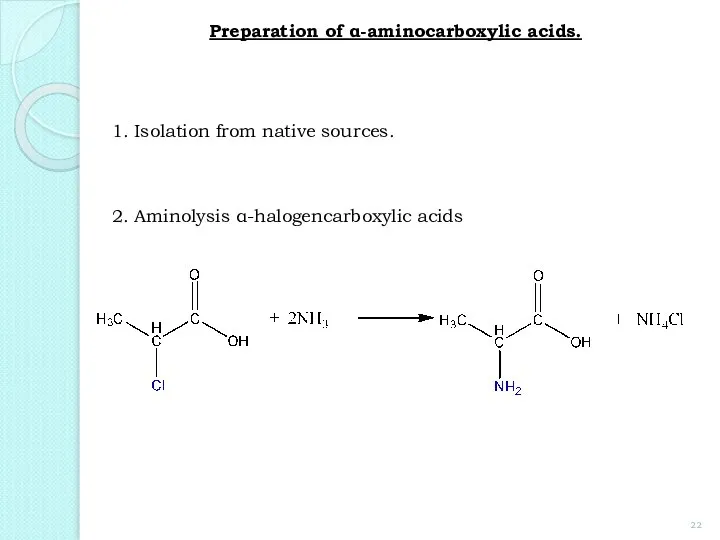
- 22. Preparation of α-aminocarboxylic acids. 2. Aminolysis α-halogencarboxylic acids 1. Isolation from native sources.
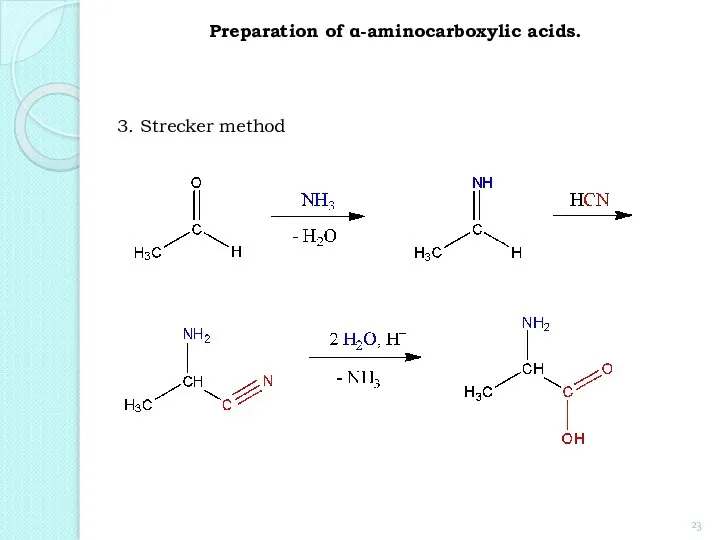
- 23. 3. Strecker method Preparation of α-aminocarboxylic acids.
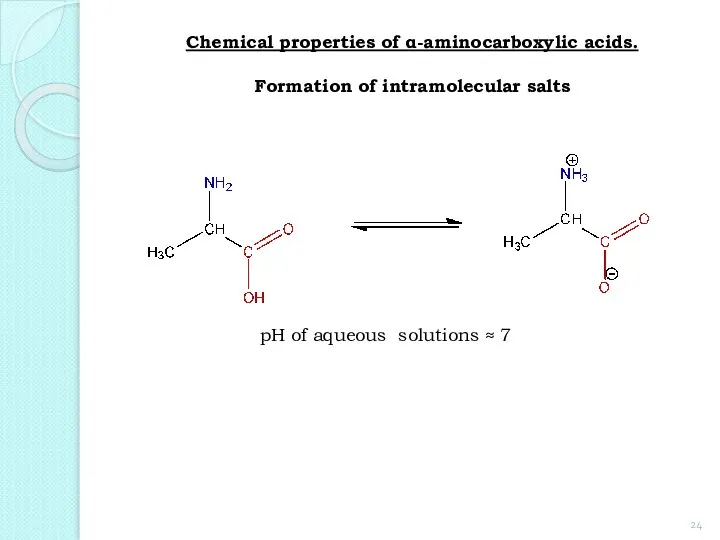
- 24. Chemical properties of α-aminocarboxylic acids. Formation of intramolecular salts pH of aqueous solutions ≈ 7
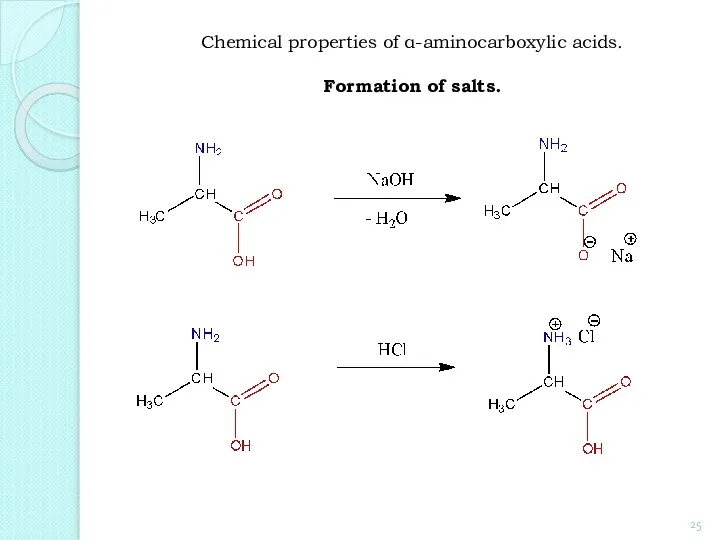
- 25. Chemical properties of α-aminocarboxylic acids. Formation of salts.
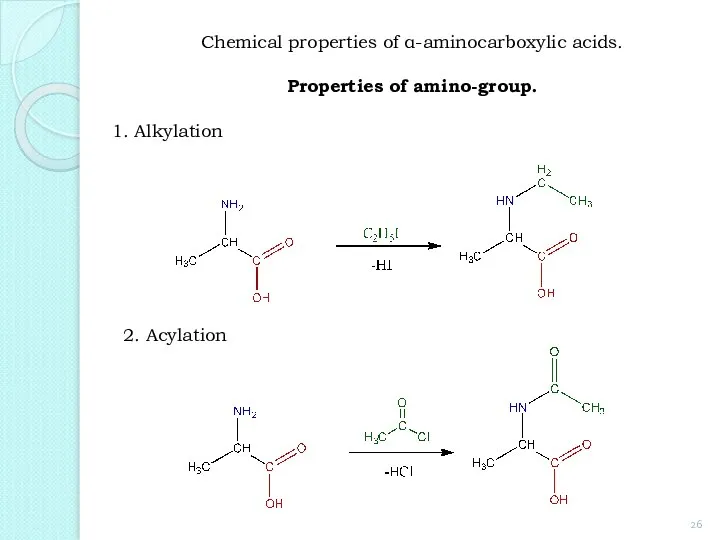
- 26. 1. Alkylation 2. Acylation Chemical properties of α-aminocarboxylic acids. Properties of amino-group.
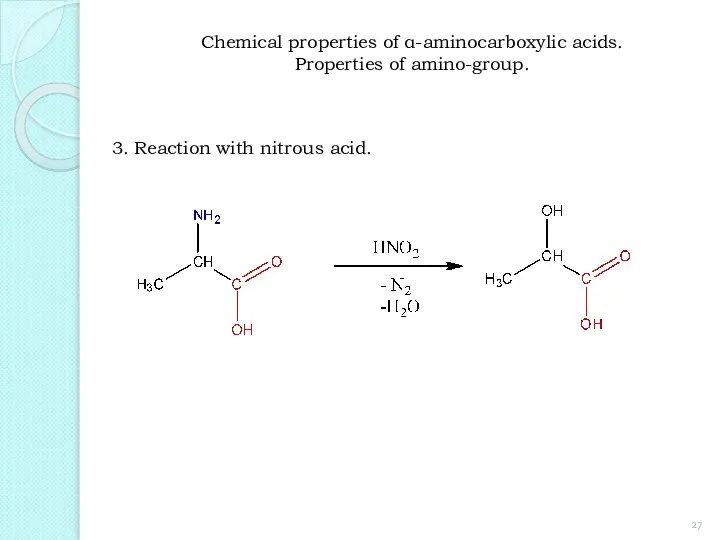
- 27. 3. Reaction with nitrous acid. Chemical properties of α-aminocarboxylic acids. Properties of amino-group.
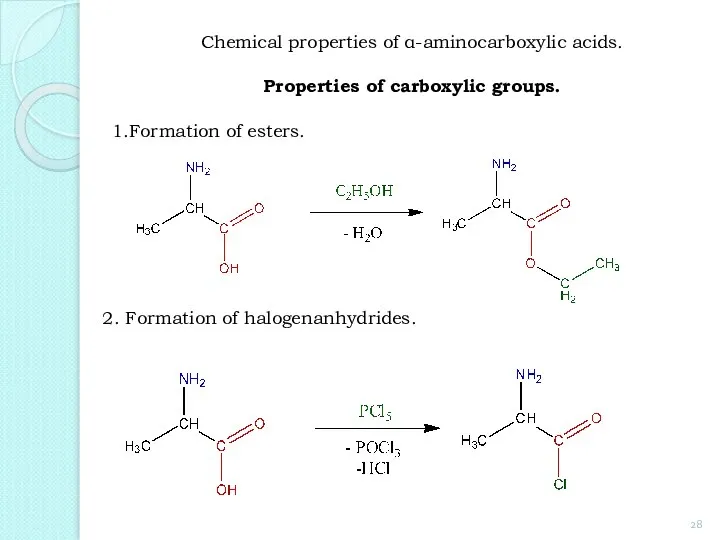
- 28. 1.Formation of esters. 2. Formation of halogenanhydrides. Chemical properties of α-aminocarboxylic acids. Properties of carboxylic groups.
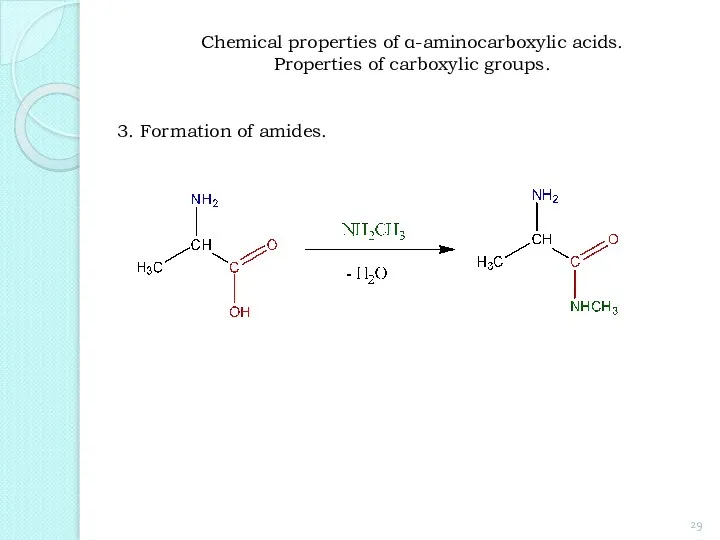
- 29. 3. Formation of amides. Chemical properties of α-aminocarboxylic acids. Properties of carboxylic groups.
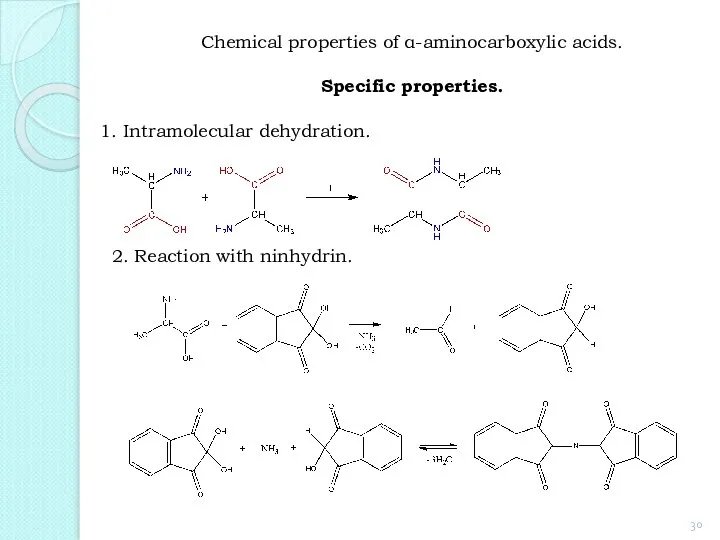
- 30. 1. Intramolecular dehydration. 2. Reaction with ninhydrin. Chemical properties of α-aminocarboxylic acids. Specific properties.
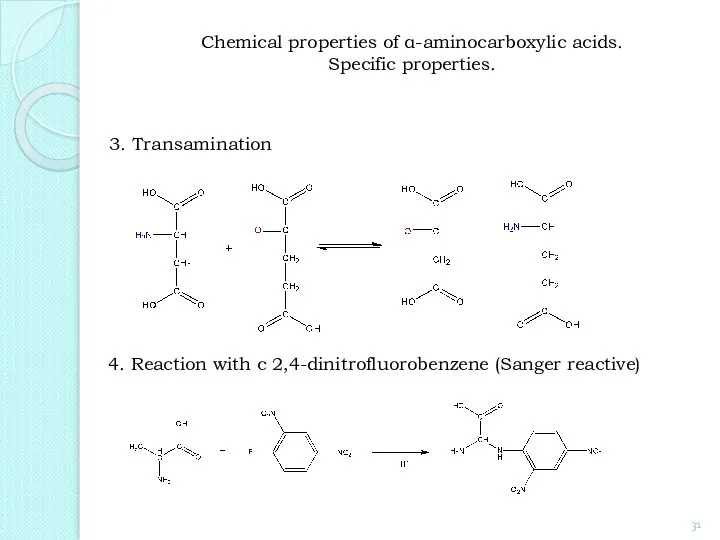
- 31. 3. Transamination 4. Reaction with с 2,4-dinitrofluorobenzene (Sanger reactive) Chemical properties of α-aminocarboxylic acids. Specific properties.
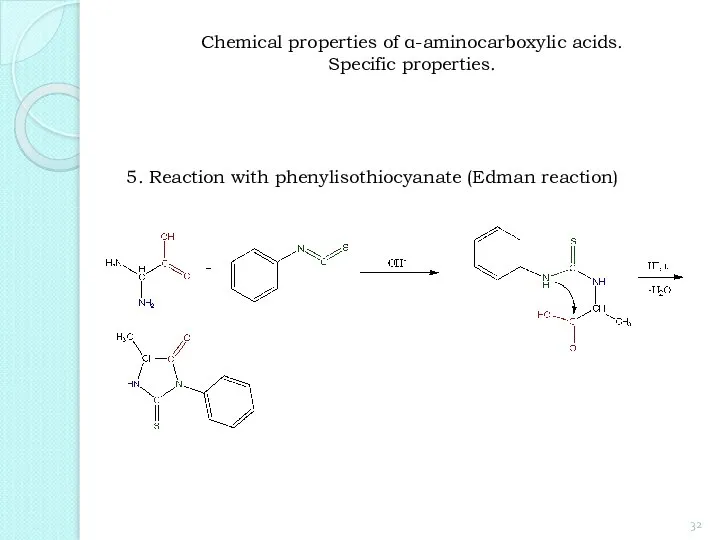
- 32. 5. Reaction with phenylisothiocyanate (Edman reaction) Chemical properties of α-aminocarboxylic acids. Specific properties.
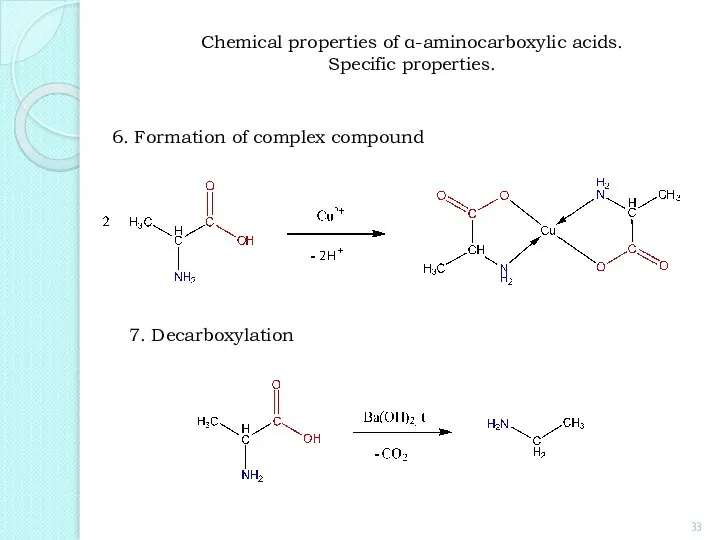
- 33. 6. Formation of complex compound 7. Decarboxylation Chemical properties of α-aminocarboxylic acids. Specific properties.
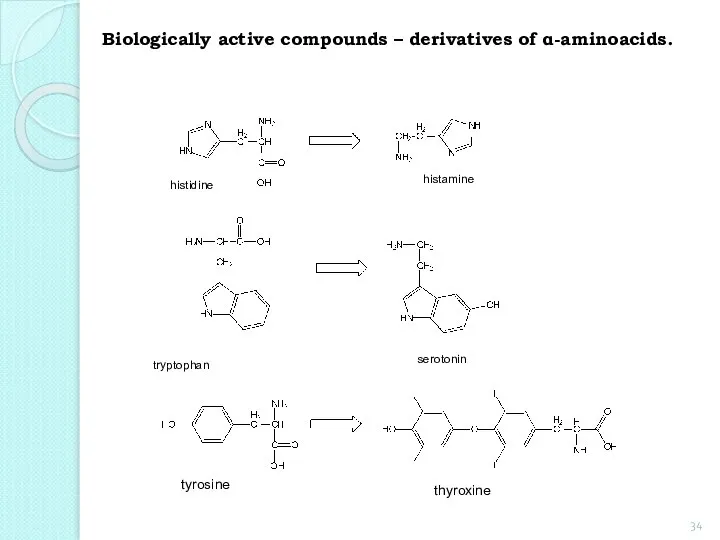
- 34. Biologically active compounds – derivatives of α-aminoacids. histidine histamine tryptophan serotonin thyroxine tyrosine
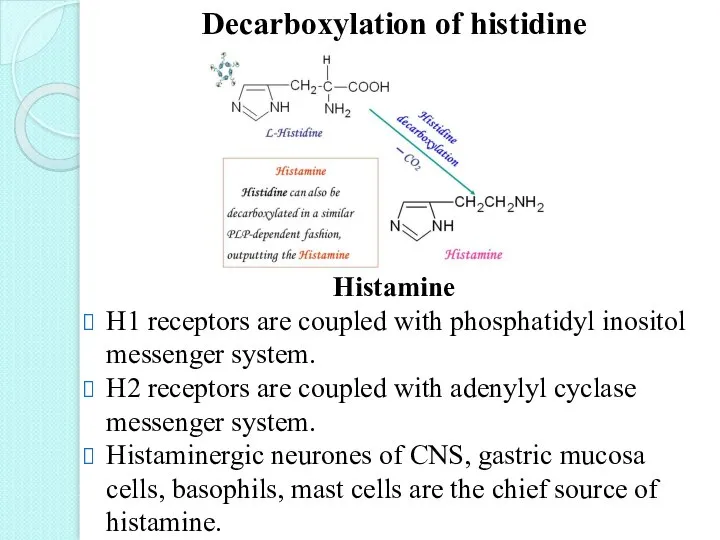
- 35. Decarboxylation of histidine Histamine H1 receptors are coupled with phosphatidyl inositol messenger system. H2 receptors are
- 36. Functions of histamine: contraction of smooth muscles of gastro-intestinal tract, bronchi; it increases HCl secretion in
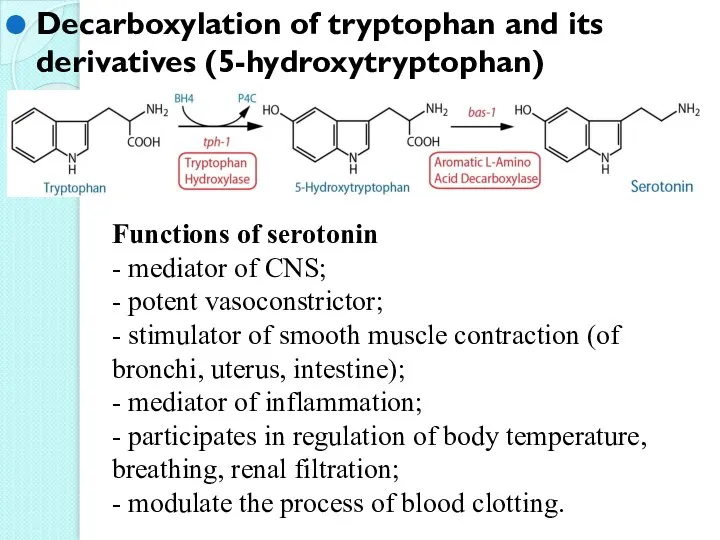
- 37. Decarboxylation of tryptophan and its derivatives (5-hydroxytryptophan) Functions of serotonin - mediator of CNS; - potent
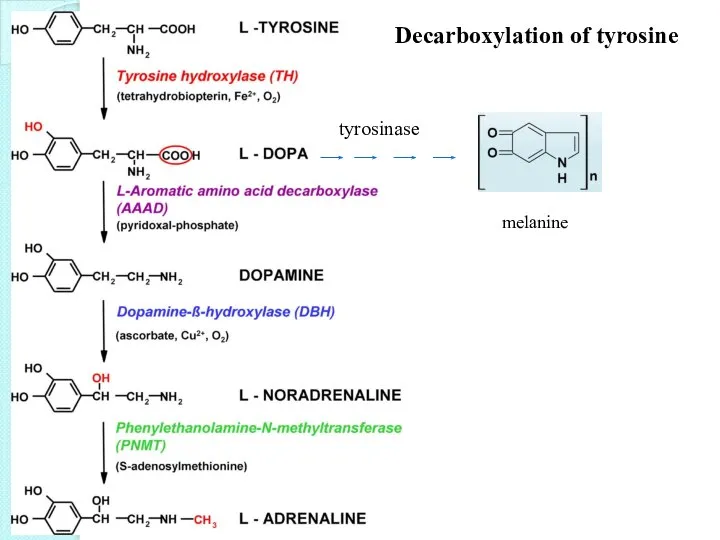
- 38. Decarboxylation of tyrosine tyrosinase melanine
- 39. Functions of epinephrine: - “fight or flight” -to increase cardiac output and to raise glucose levels
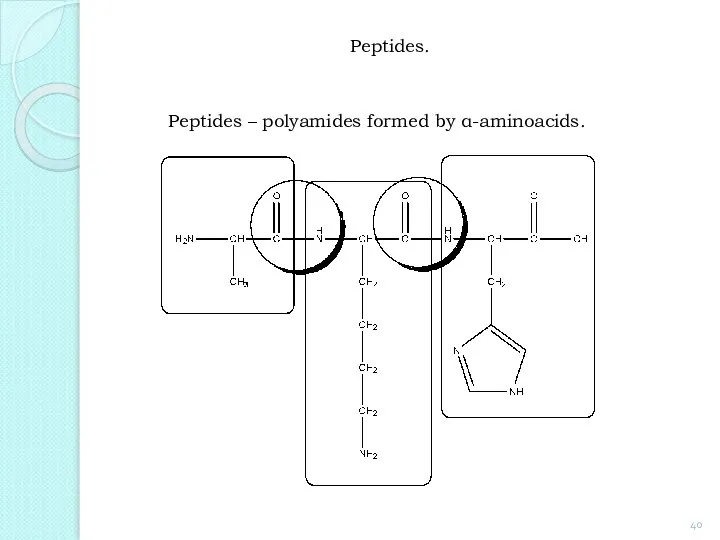
- 40. Peptides. Peptides – polyamides formed by α-aminoacids.
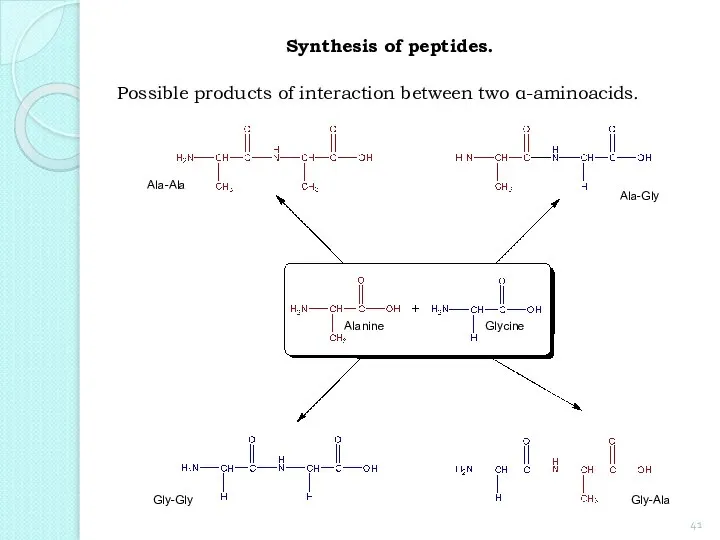
- 41. Synthesis of peptides. Possible products of interaction between two α-aminoacids. Alanine Glycine Ala-Ala Ala-Gly Gly-Gly Gly-Ala
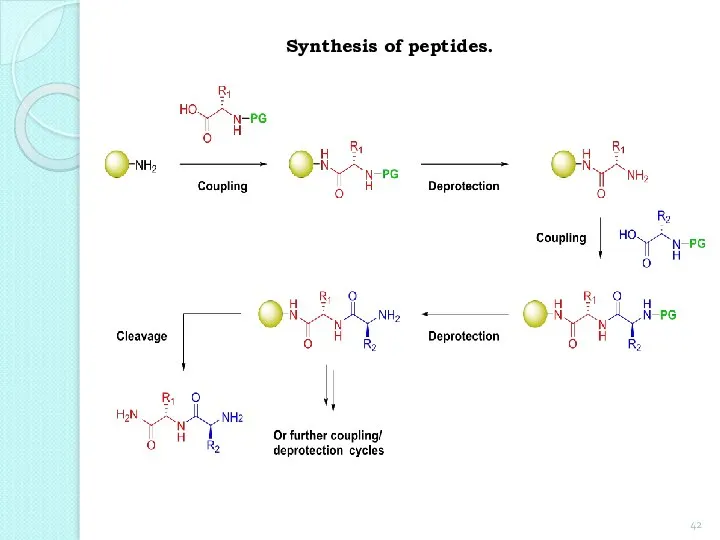
- 42. Synthesis of peptides.
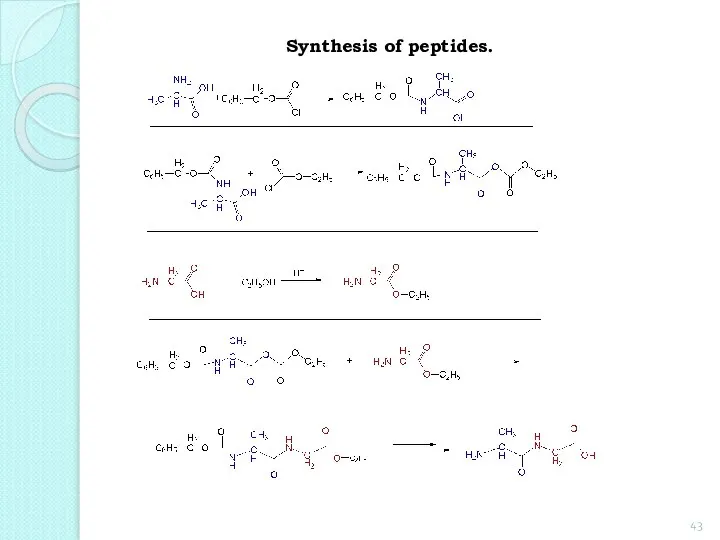
- 43. Synthesis of peptides.
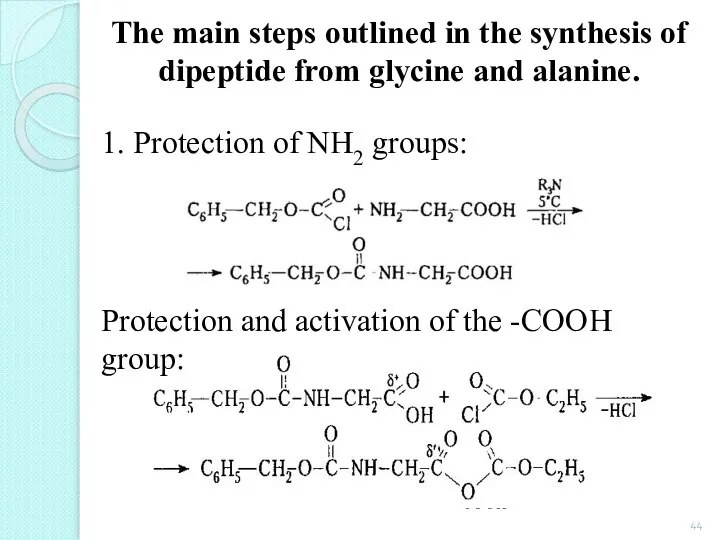
- 44. The main steps outlined in the synthesis of dipeptide from glycine and alanine. 1. Protection of
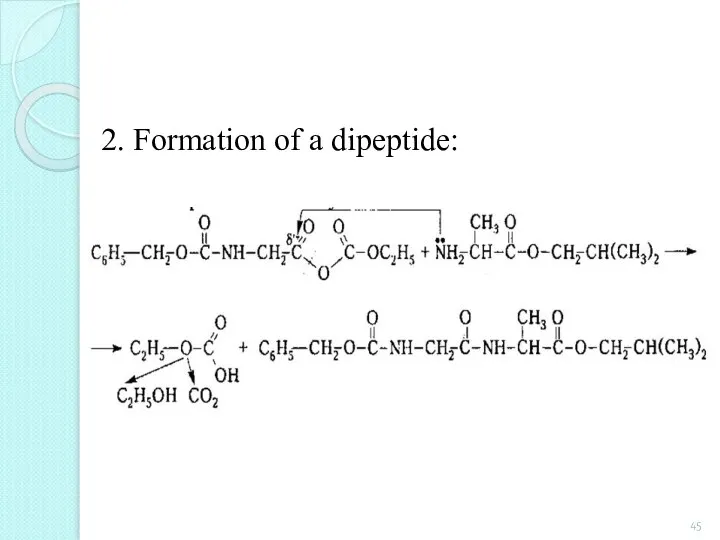
- 45. 2. Formation of a dipeptide:
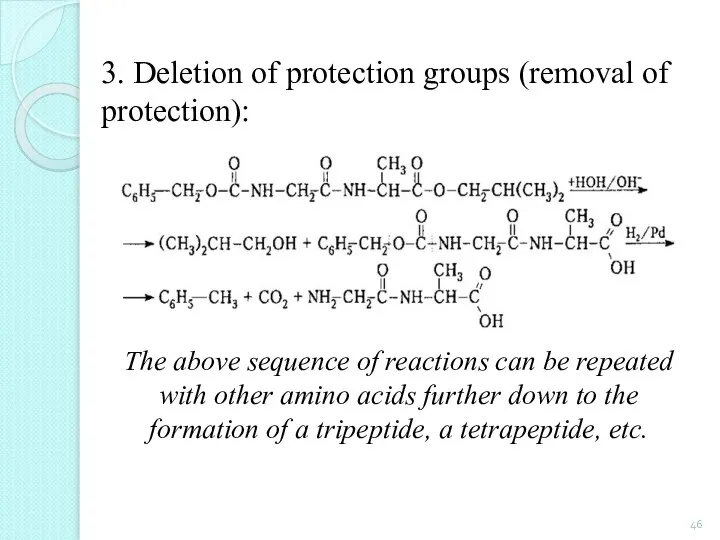
- 46. 3. Deletion of protection groups (removal of protection): The above sequence of reactions can be repeated
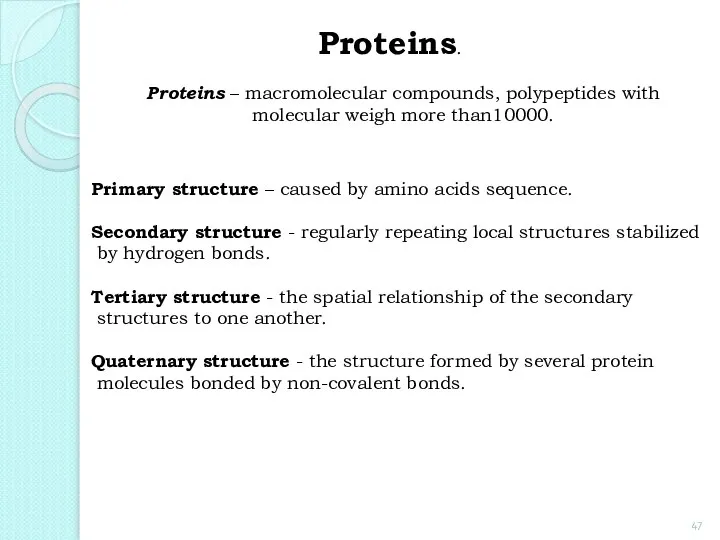
- 47. Proteins. Proteins – macromolecular compounds, polypeptides with molecular weigh more than10000. Primary structure – caused by
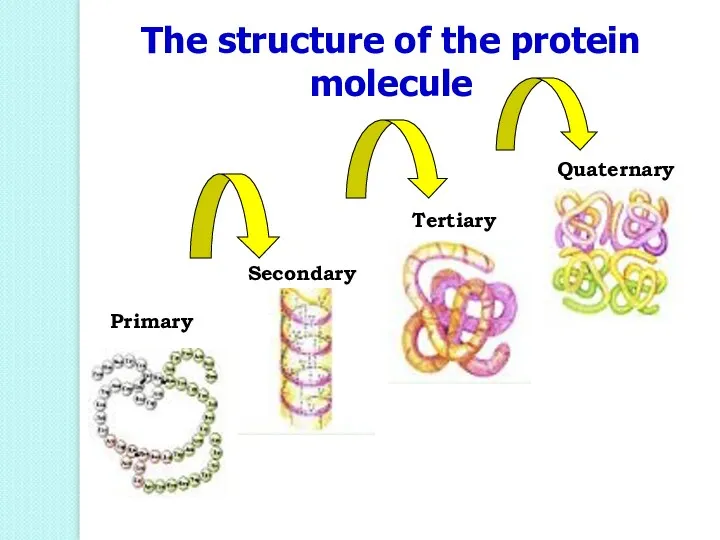
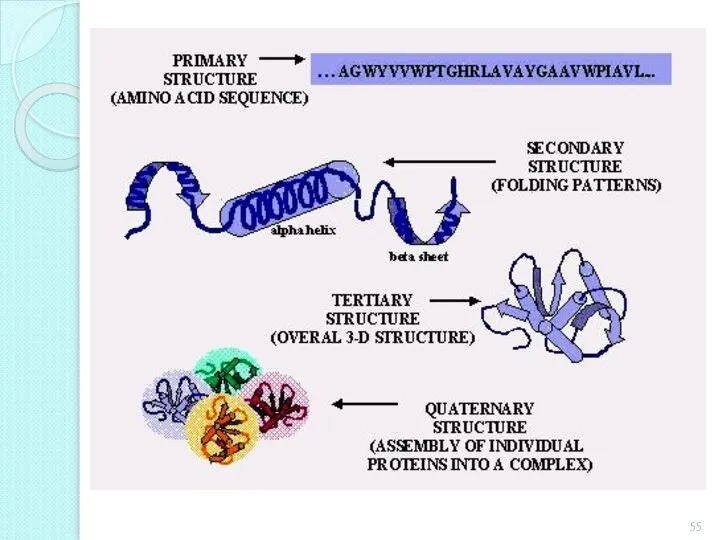
- 48. The structure of the protein molecule Primary Secondary Tertiary Quaternary
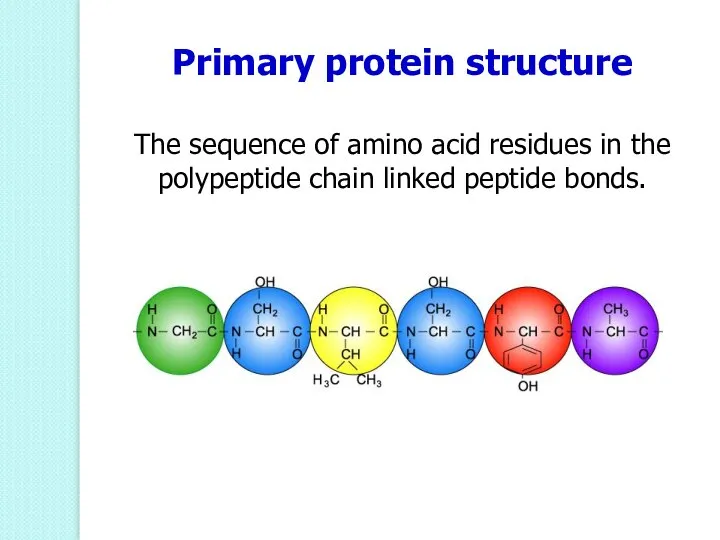
- 49. Primary protein structure The sequence of amino acid residues in the polypeptide chain linked peptide bonds.
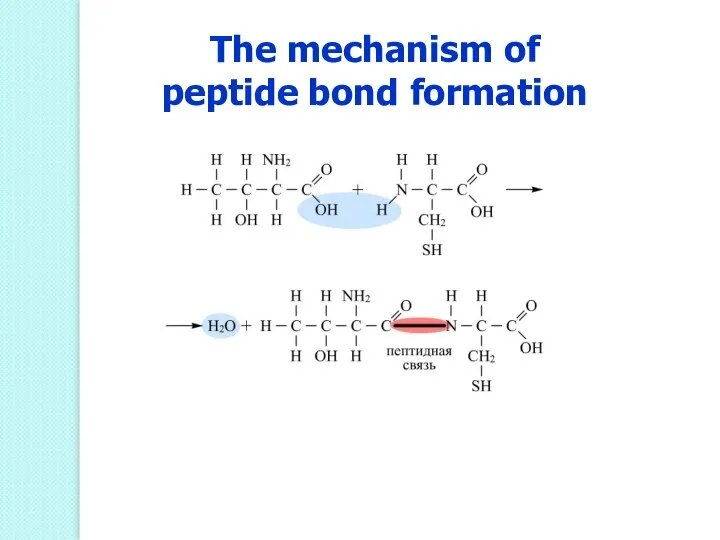
- 50. The mechanism of peptide bond formation
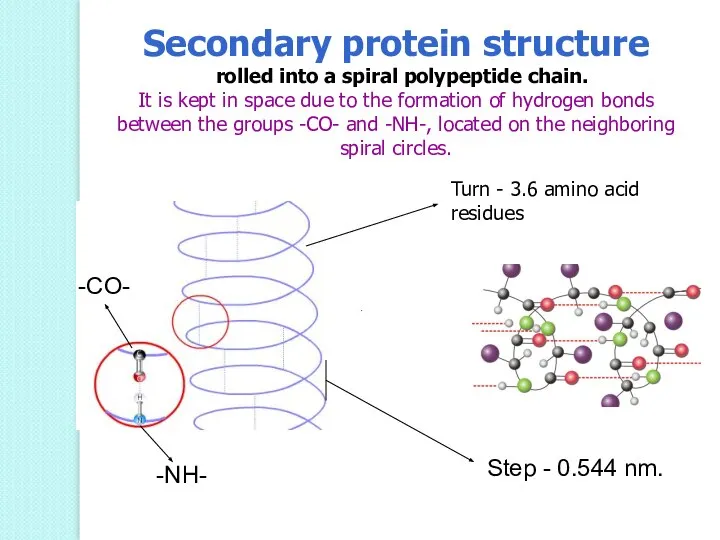
- 51. Turn - 3.6 amino acid residues Step - 0.544 nm. -CO- -NH- Secondary protein structure rolled
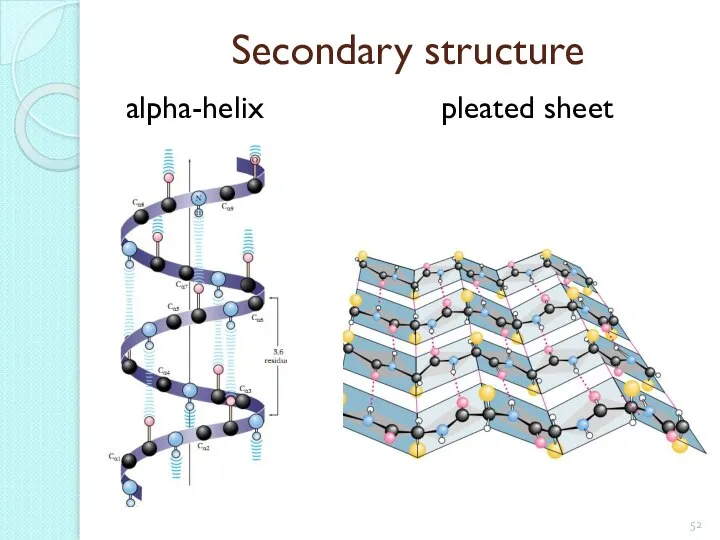
- 52. Secondary structure alpha-helix pleated sheet
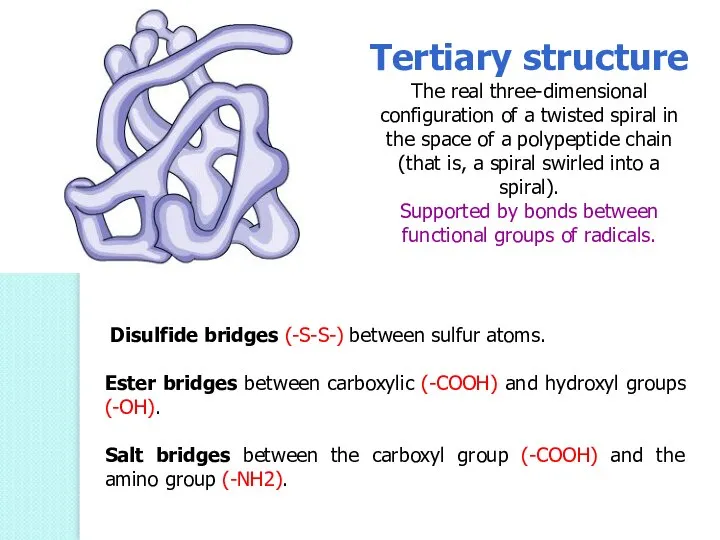
- 53. Tertiary structure The real three-dimensional configuration of a twisted spiral in the space of a polypeptide
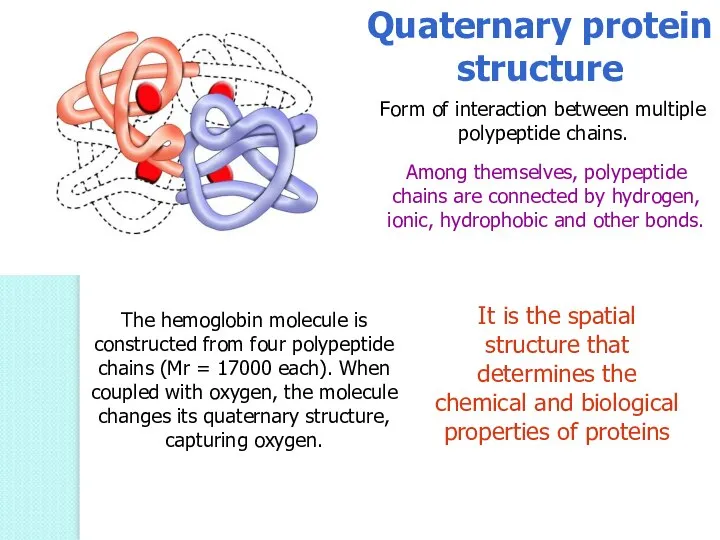
- 54. Quaternary protein structure Form of interaction between multiple polypeptide chains. Among themselves, polypeptide chains are connected
- 57. Скачать презентацию



 Неметаллы в природе и жизни человека
Неметаллы в природе и жизни человека Моющие и чистящие средства
Моющие и чистящие средства Реакции, подтверждающие взаимосвязь различных
Реакции, подтверждающие взаимосвязь различных Природный газ. Алканы
Природный газ. Алканы Презентация на тему Витамин Е
Презентация на тему Витамин Е  Аммиак. Свойства (9 класс)
Аммиак. Свойства (9 класс) Явление хемилюменисценции
Явление хемилюменисценции Атом. Изотопы
Атом. Изотопы Сокровища соляных дворцов
Сокровища соляных дворцов Классы неорганических соединений
Классы неорганических соединений Углеводороды
Углеводороды Кристаллы. Формирование кристаллов
Кристаллы. Формирование кристаллов Получение азокрасителя в лабораторных условиях
Получение азокрасителя в лабораторных условиях Які існують способи розділення сумішей?
Які існують способи розділення сумішей? Таблица Д.И. Менделеева
Таблица Д.И. Менделеева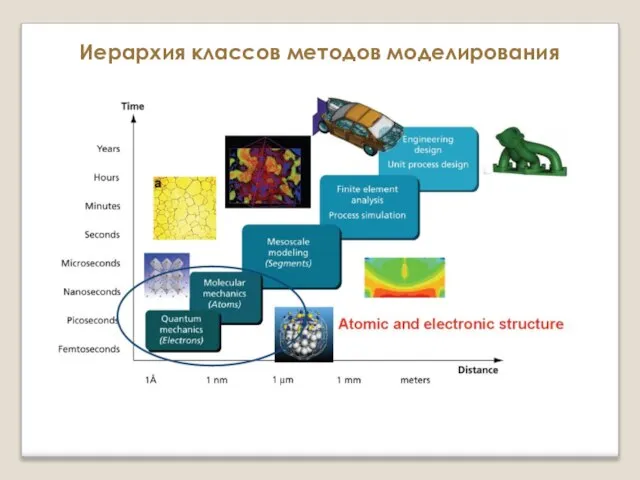 Поверхность потенциальной энергии и расчет сил, действующих на ионы
Поверхность потенциальной энергии и расчет сил, действующих на ионы Химические свойства бензола. Получение, применение. Учитель биологии-химии МОУ «СОШ р.п. Озинки» Хорова Людмила Владимировна
Химические свойства бензола. Получение, применение. Учитель биологии-химии МОУ «СОШ р.п. Озинки» Хорова Людмила Владимировна Щелочноземельные металлы
Щелочноземельные металлы Понятие Эффективность. Принцип Ле-Шателье
Понятие Эффективность. Принцип Ле-Шателье Химия и география. Викторина
Химия и география. Викторина Металлы и их соединения
Металлы и их соединения Лекция_классы_неогранических_соединений
Лекция_классы_неогранических_соединений Производство метанола
Производство метанола Основные классы неорганических соединений
Основные классы неорганических соединений Обмен углеводов
Обмен углеводов Номенклатура и изомерия аренов
Номенклатура и изомерия аренов Марганец. Химия элементов. Кислородосодержащие соединения
Марганец. Химия элементов. Кислородосодержащие соединения Презентация на тему Вопросы от …
Презентация на тему Вопросы от …