Содержание
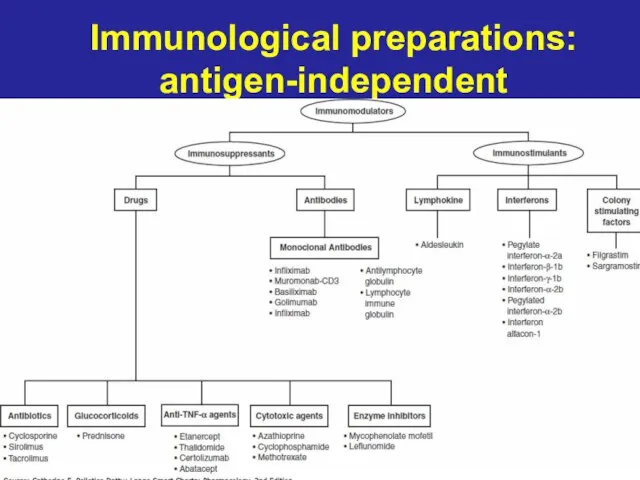
- 2. Immunological preparations: antigen-independent
- 3. Immunological preparations: antigen dependent -Vaccines (antigens) -Immune sera or Immunoglobulins
- 5. Immunization is the method of controlling infections Immune responses to immunization or immuno-therapy can block the
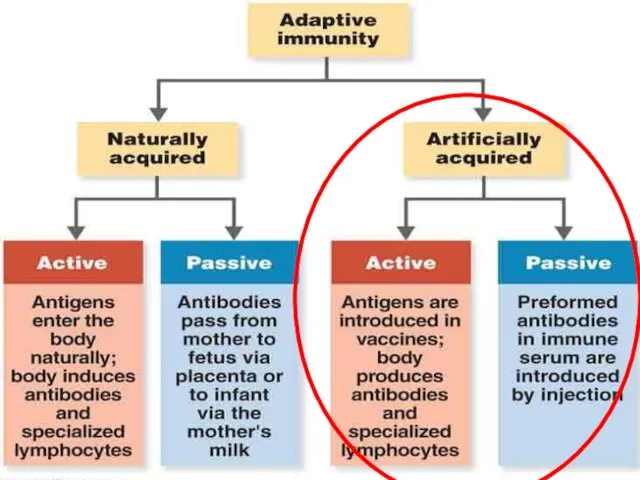
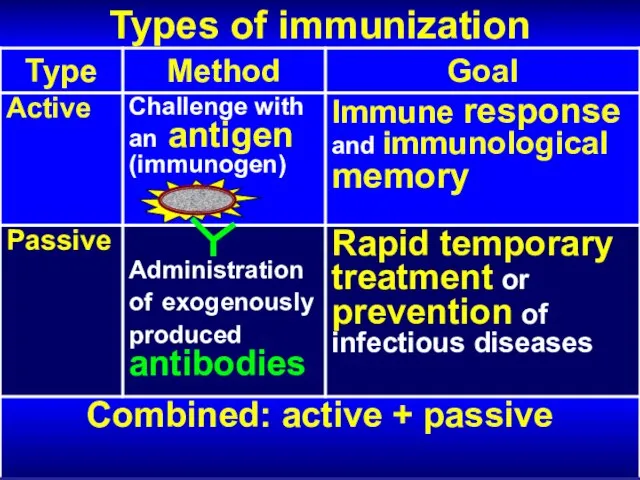
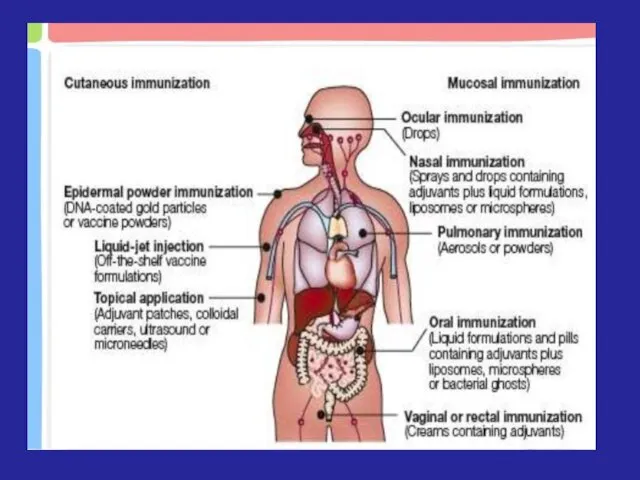
- 6. Types of immunization
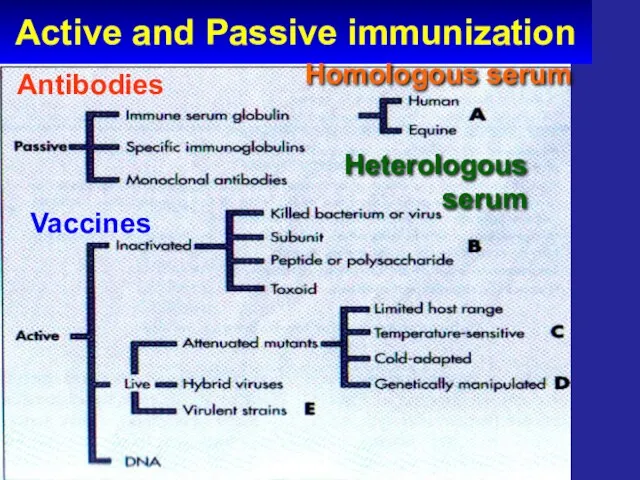
- 7. Active and Passive immunization Vaccines
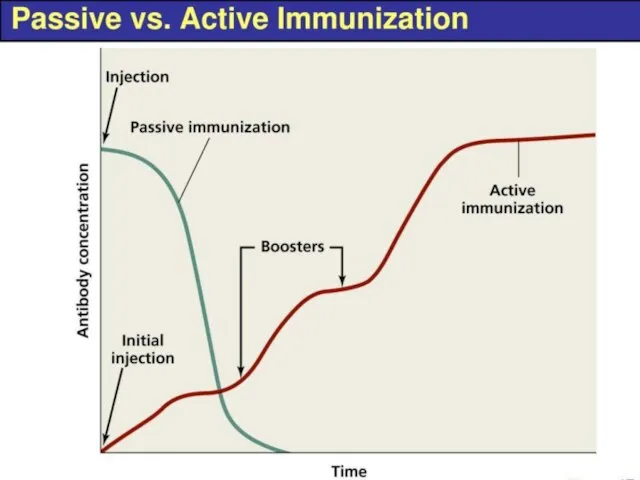
- 8. Active and Passive immunization Antibodies Vaccines Homologous serum Heterologous serum
- 9. Artificial passive immunization (API) API may be used: To prevent disease after a known exposure (needle
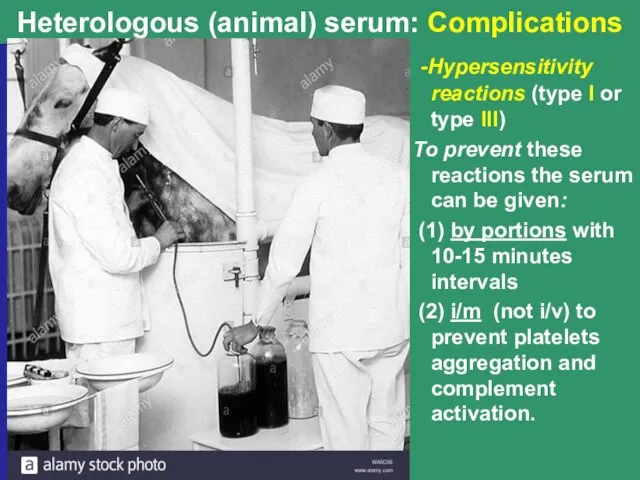
- 10. Heterologous (animal) serum: Complications -Hypersensitivity reactions (type I or type III) To prevent these reactions the
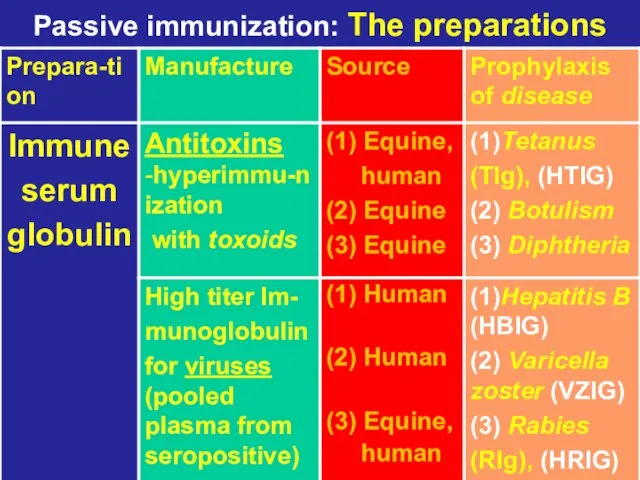
- 11. Passive immunization: The preparations
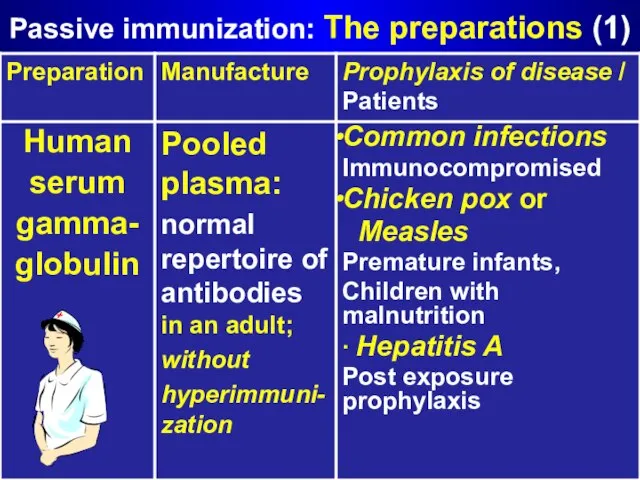
- 12. Passive immunization: The preparations (1)
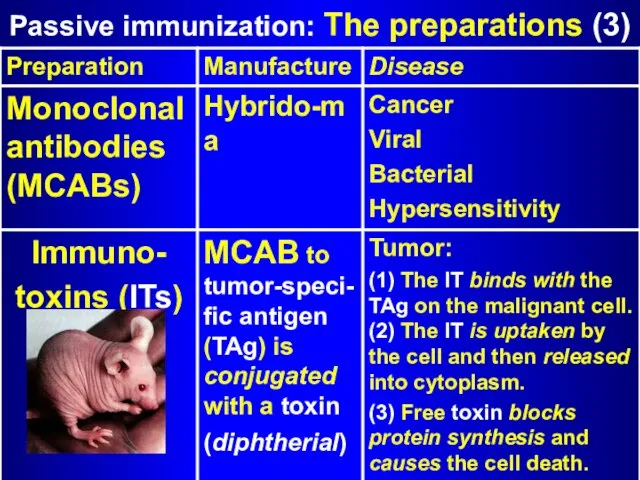
- 13. Passive immunization: The preparations (3)
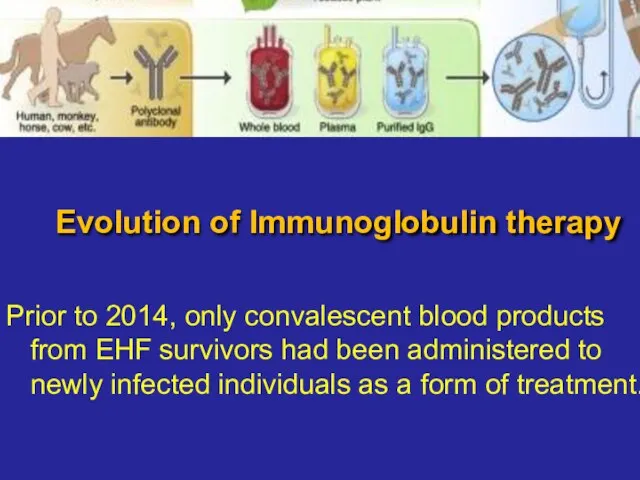
- 14. Evolution of Immunoglobulin therapy Prior to 2014, only convalescent blood products from EHF survivors had been
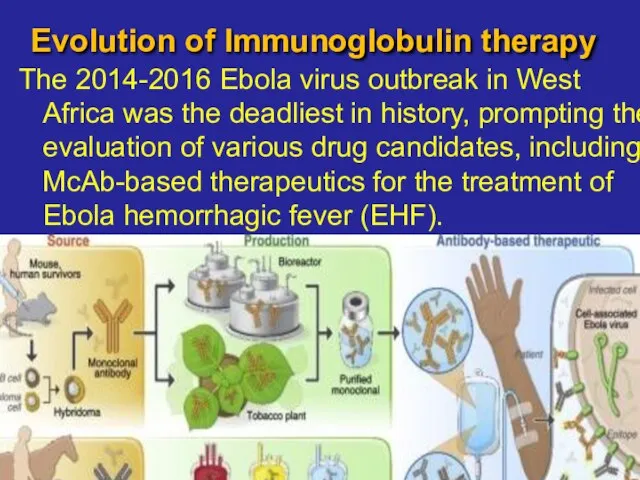
- 15. Evolution of Immunoglobulin therapy The 2014-2016 Ebola virus outbreak in West Africa was the deadliest in
- 16. Evolution of Immunoglobulin therapy the genes encoding for the antibodies were extracted from the hybridomas, genetically
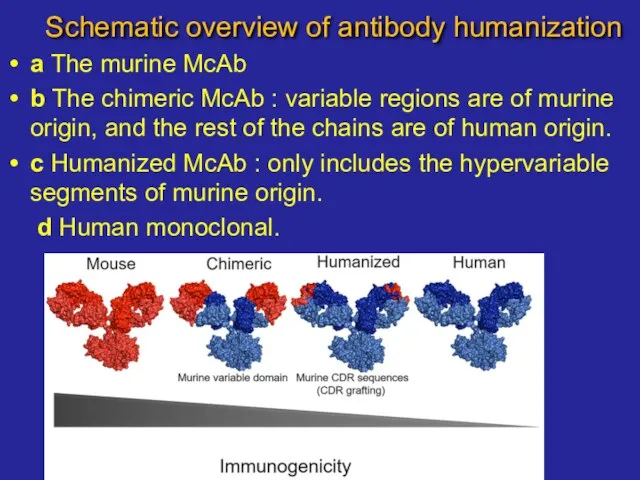
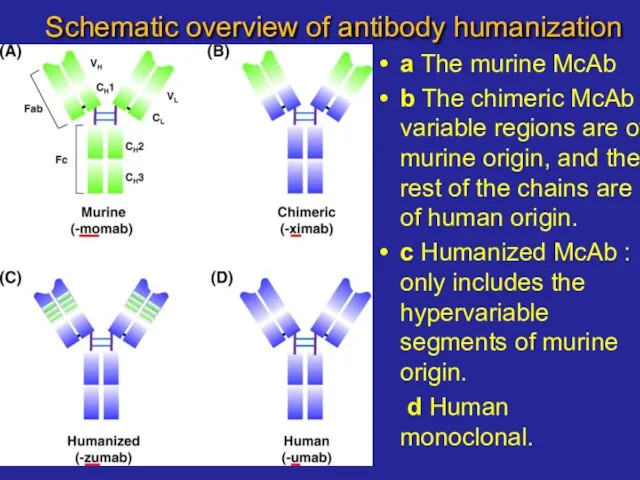
- 17. Schematic overview of antibody humanization a The murine McAb b The chimeric McAb : variable regions
- 18. Schematic overview of antibody humanization a The murine McAb b The chimeric McAb : variable regions
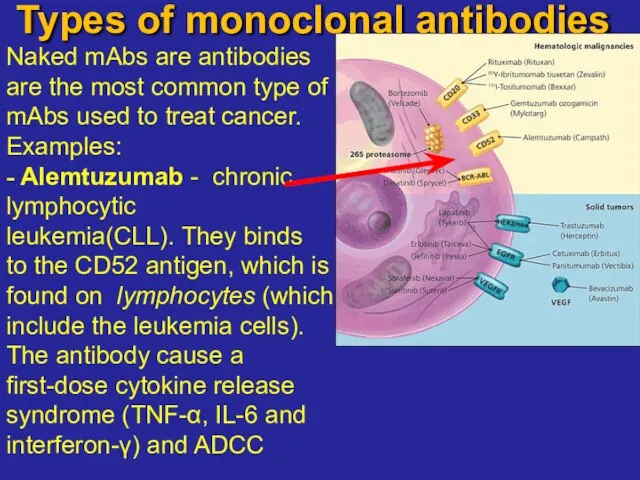
- 19. Types of monoclonal antibodies Naked mAbs are antibodies are the most common type of mAbs used
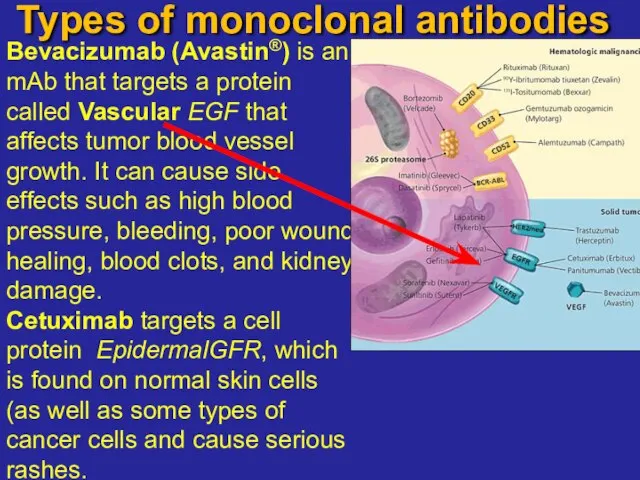
- 20. Types of monoclonal antibodies endothelial growth factor Bevacizumab (Avastin®) is an mAb that targets a protein
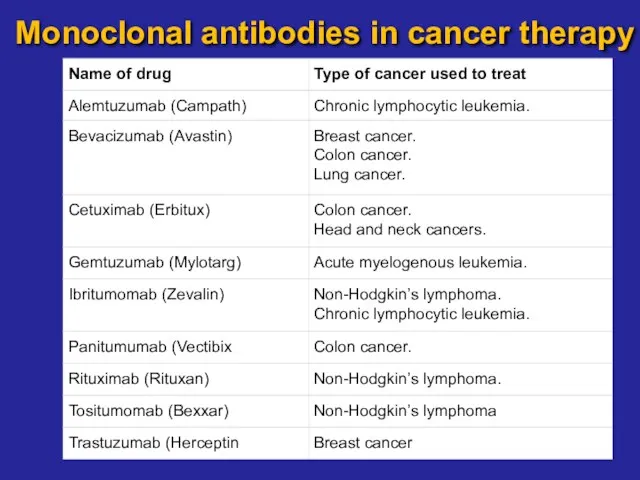
- 21. Monoclonal antibodies in cancer therapy
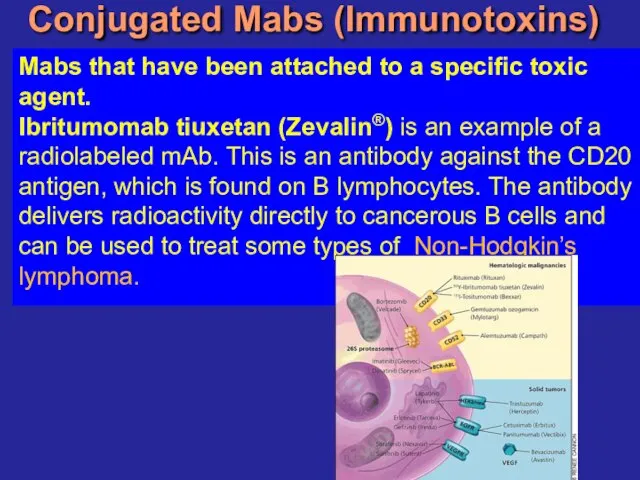
- 22. Conjugated Mabs (Immunotoxins) Mabs that have been attached to a specific toxic agent. Ibritumomab tiuxetan (Zevalin®)
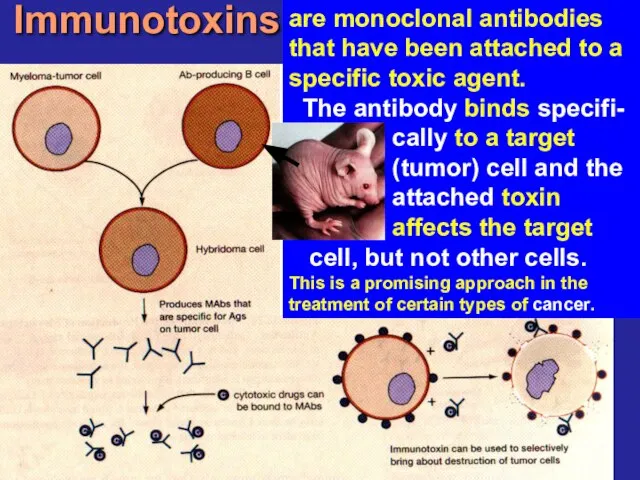
- 23. Immunotoxins are monoclonal antibodies that have been attached to a specific toxic agent. The antibody binds
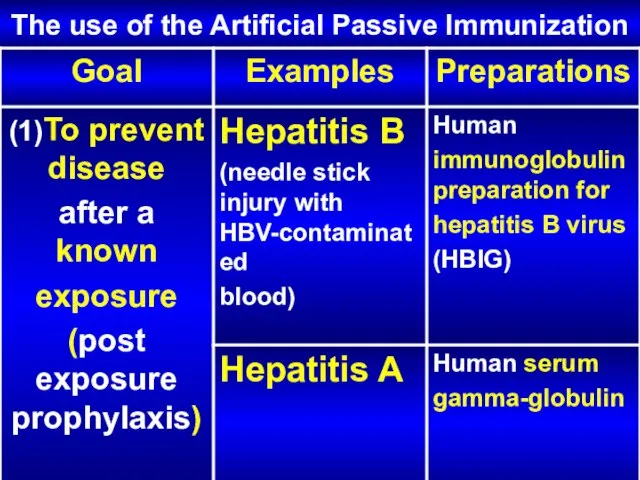
- 24. The use of the Artificial Passive Immunization
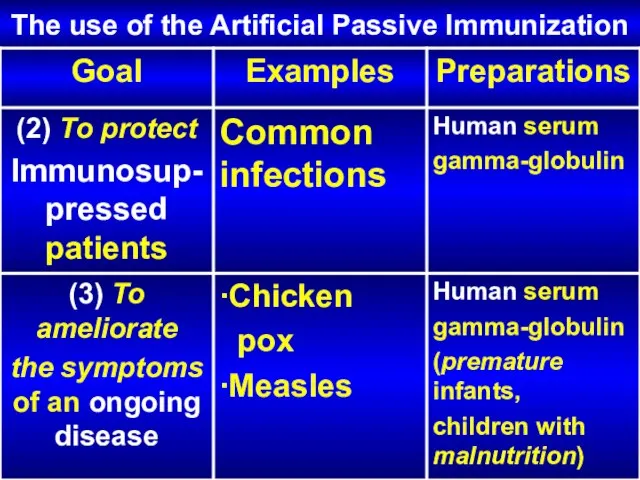
- 25. The use of the Artificial Passive Immunization
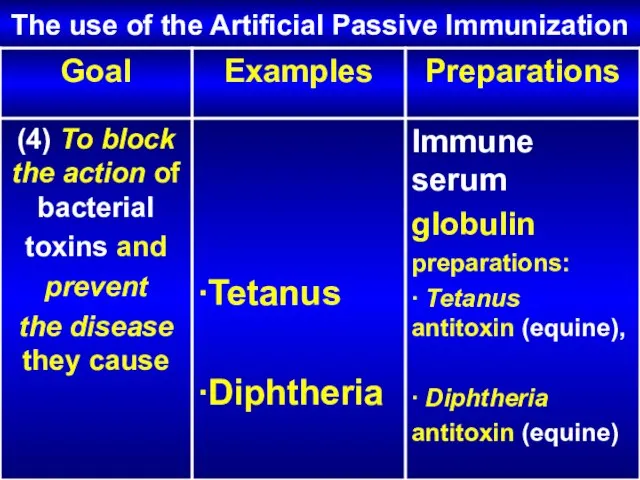
- 26. The use of the Artificial Passive Immunization
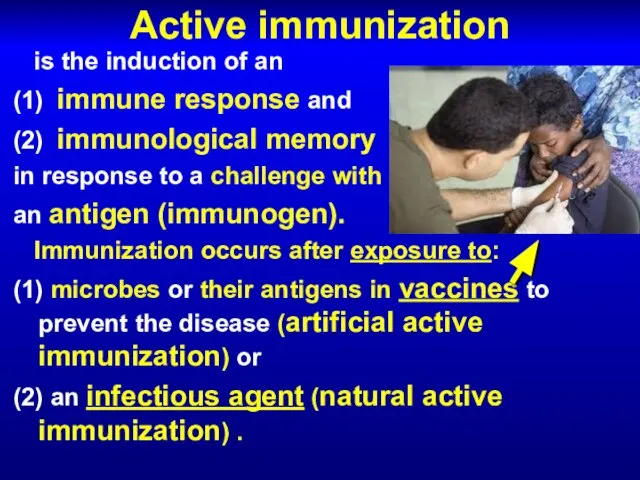
- 27. Active immunization is the induction of an (1) immune response and (2) immunological memory in response
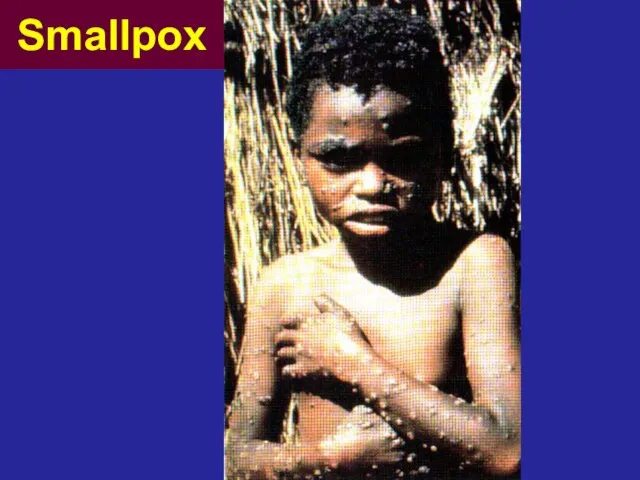
- 28. The term ‘vaccine’ (Latin ‘vacca’, cow) This term comes from the first successful immunization against smallpox
- 29. Smallpox
- 30. Vaccination is the artificial active immunization Louis Pasteur introduced this term recognizing the relevance of Jenner’s
- 31. An immunizing agent derived from microorganism is called vaccine A vaccine consists either of whole organism
- 32. - Conventional vaccines - usually contain inactivated disease-causing organisms or proteins made by the pathogen (antigens),
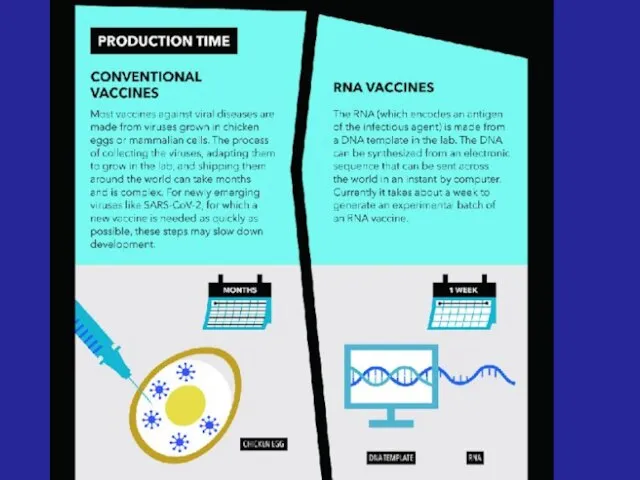
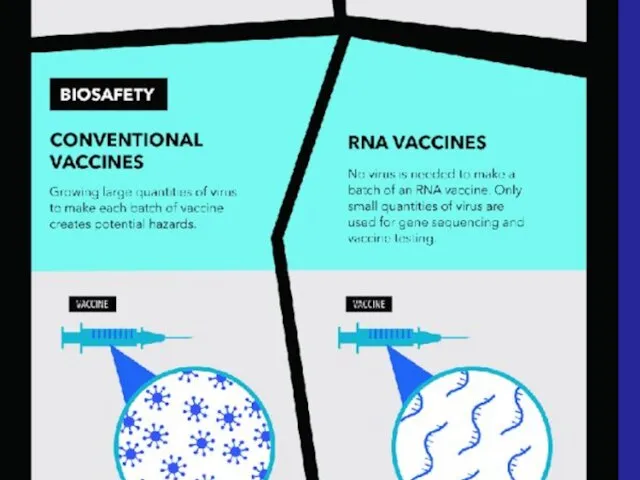
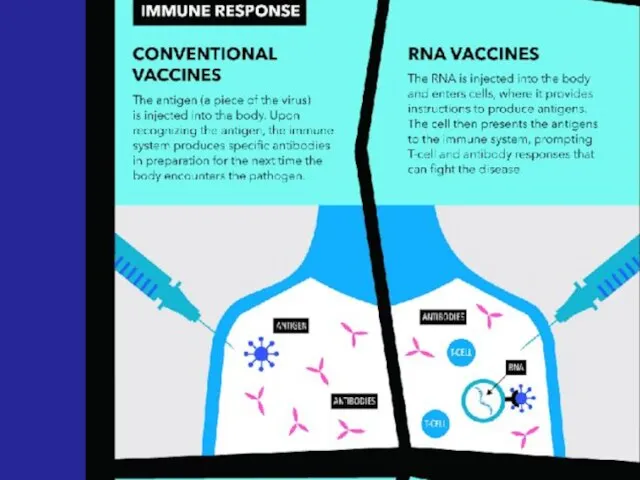
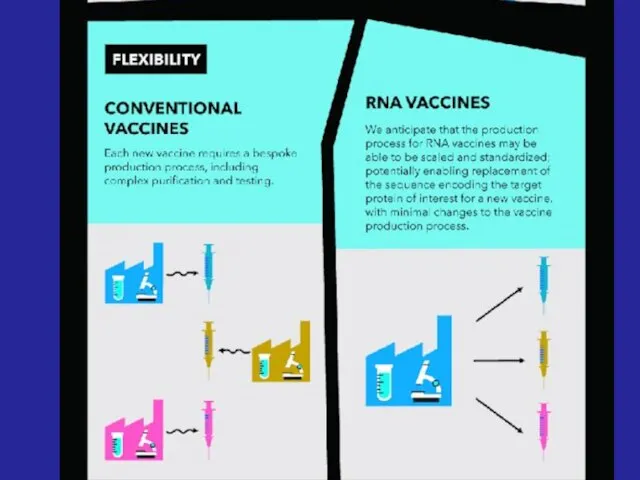
- 33. - Advanced vaccines - RNA vaccines use a different : RNA vaccine consists of an mRNA
- 38. Types of Live Vaccines (LVNs) LVNs are prepared with organisms limited in the ability to cause
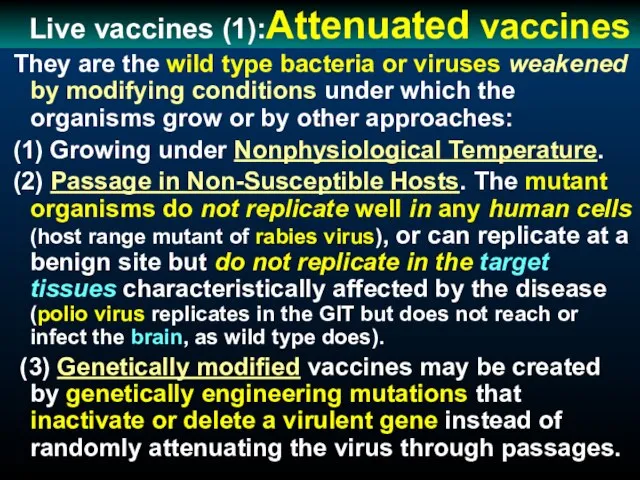
- 39. Live vaccines (1):Attenuated vaccines They are the wild type bacteria or viruses weakened by modifying conditions
- 40. Live vaccines (2):Attenuated vaccines Generally attenuation can be achieved by modify-ing conditions under which the organism
- 41. Live viral vaccines (LVVNs): Immune responses Immunization with a LVVNs resembles natural infection and elicits both
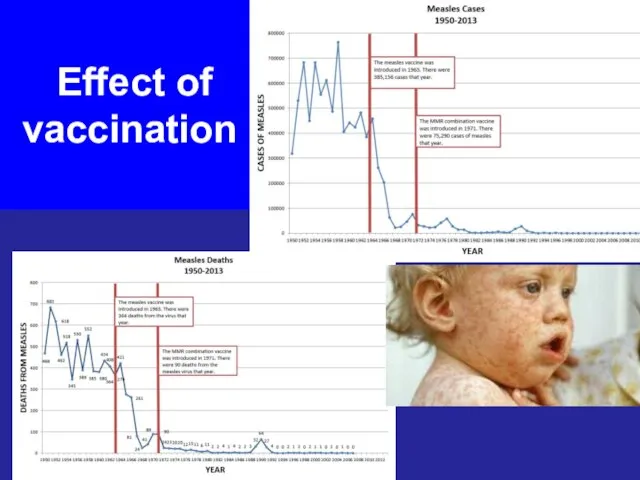
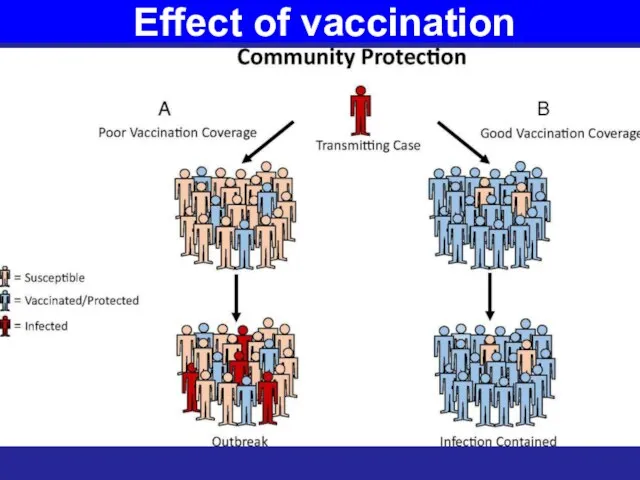
- 42. Effect of vaccination
- 43. Effect of vaccination
- 44. The Anti-vaccination Movement: A Regression in Modern Medicine
- 45. The Anti-vaccination Movement: A Regression in Modern Medicine There have been recent trends of parents in
- 46. The Anti-vaccination Movement: A Regression in Modern Medicine Almost incredibly, the trigger for what would become
- 47. The Anti-vaccination Movement: A Regression in Modern Medicine It reported on the cases of 12 anonymous
- 48. Live divergent vaccines: (2) Virulent micro-organisms from other species that share antigens with human pathogens: (1)
- 49. Newborn baby Rotaviruses vaccine immunization Rotavirus is a virus that causes diarrhea, mostly in babies and
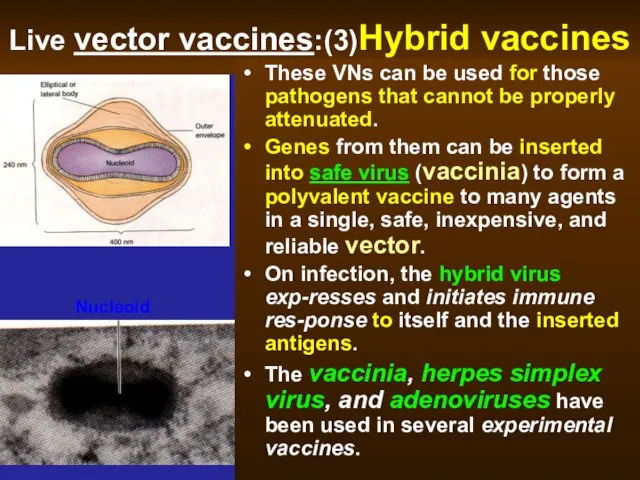
- 50. Live vector vaccines:(3)Hybrid vaccines These VNs can be used for those pathogens that cannot be properly
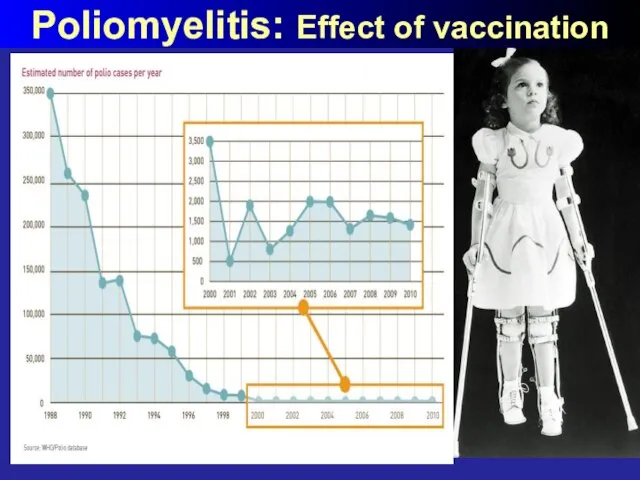
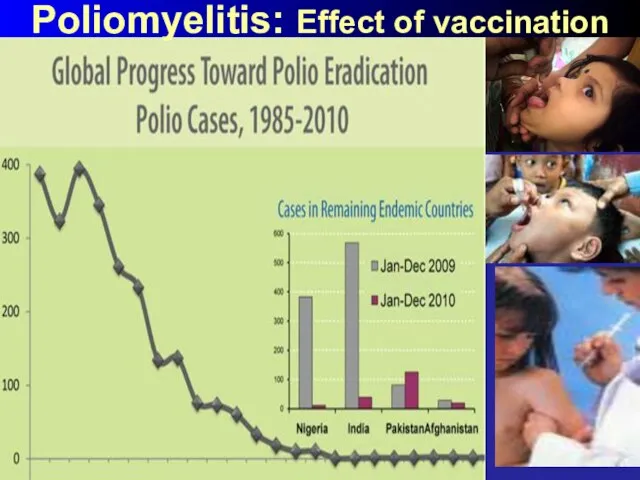
- 52. Poliomyelitis: Effect of vaccination
- 53. Poliomyelitis: Effect of vaccination
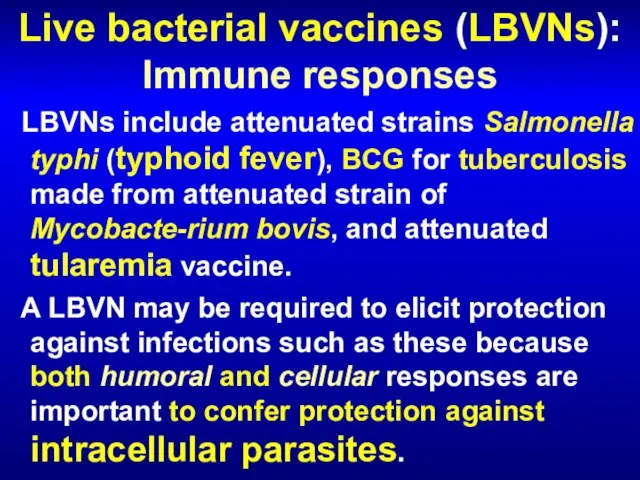
- 54. Live bacterial vaccines (LBVNs): Immune responses LBVNs include attenuated strains Salmonella typhi (typhoid fever), BCG for
- 55. Live vaccines: The advantages (1) The immunity is long live, and mimics the normal immune responses.
- 56. Live vaccines: The disadvantages (1) they may cause disease in immunosuppressed individuals and should be replaced
- 57. Inactivated vaccines (IVNs) IVNs provide safe antigen for immunization and are used to confer protection against
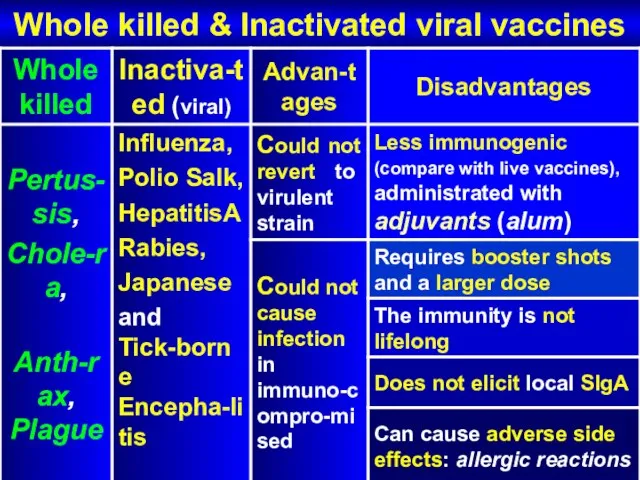
- 58. Whole killed & Inactivated viral vaccines
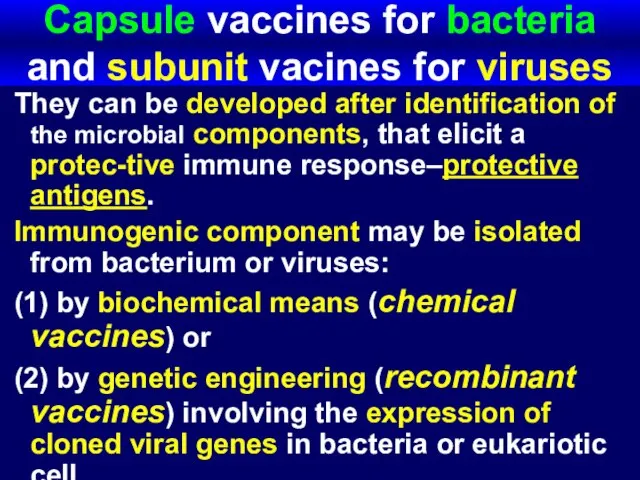
- 59. Capsule vaccines for bacteria and subunit vacines for viruses They can be developed after identification of
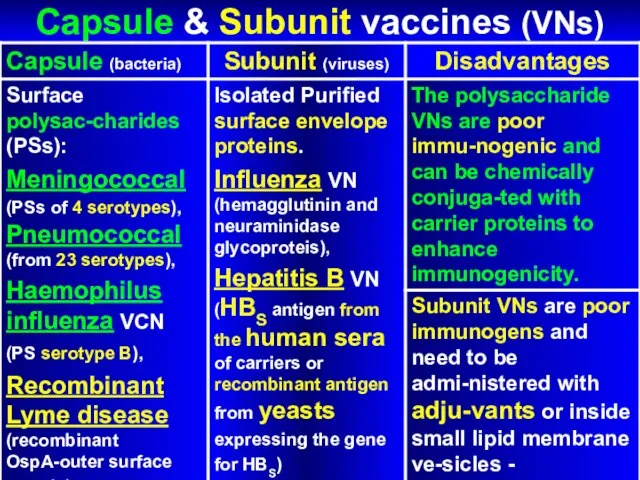
- 60. Capsule & Subunit vaccines (VNs)
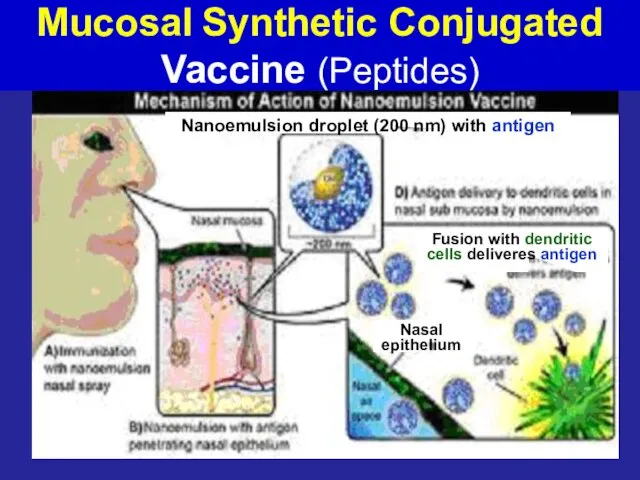
- 61. Mucosal Synthetic Conjugated Vaccine (Peptides) Nanoemulsion droplet (200 nm) with antigen Fusion with dendritic cells deliveres
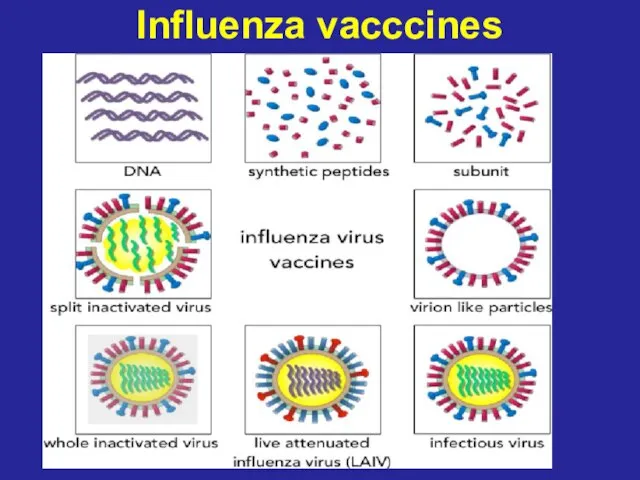
- 62. Influenza vacccines
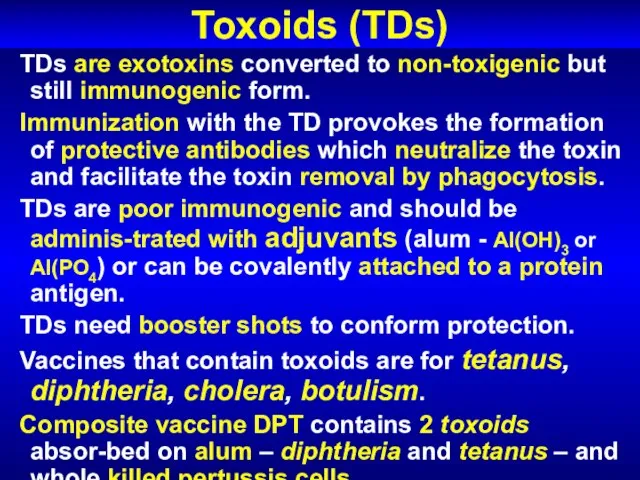
- 63. Toxoids (TDs) TDs are exotoxins converted to non-toxigenic but still immunogenic form. Immunization with the TD
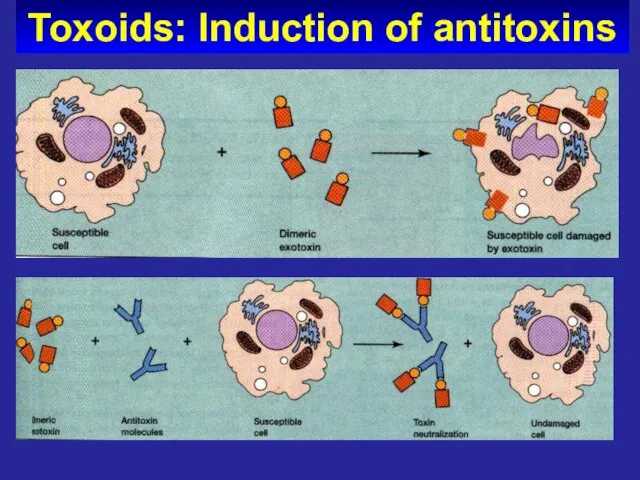
- 64. Toxoids: Induction of antitoxins
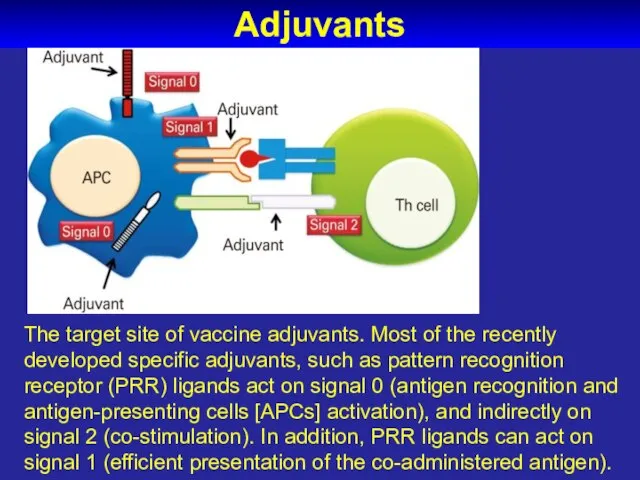
- 65. Adjuvants The target site of vaccine adjuvants. Most of the recently developed specific adjuvants, such as
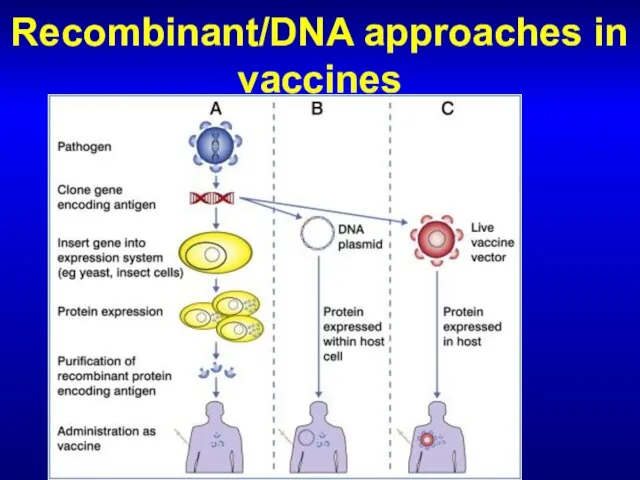
- 66. Recombinant/DNA approaches in vaccines
- 67. DNA vaccines DNA vaccines consist of naked DNA code for a gene for vaccinal protective antigen.
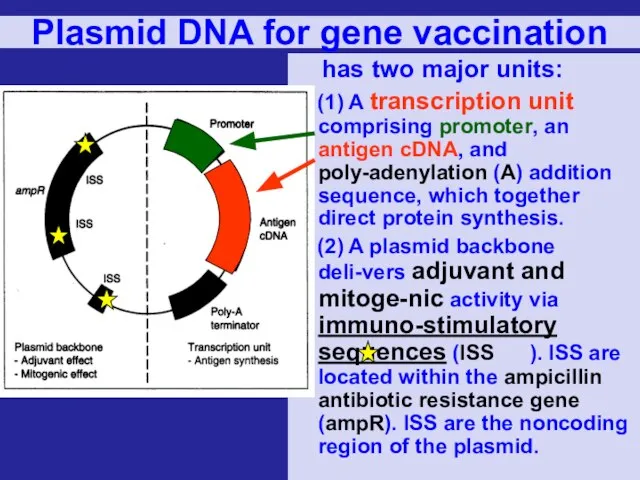
- 68. Plasmid DNA for gene vaccination has two major units: (1) A transcription unit comprising promoter, an
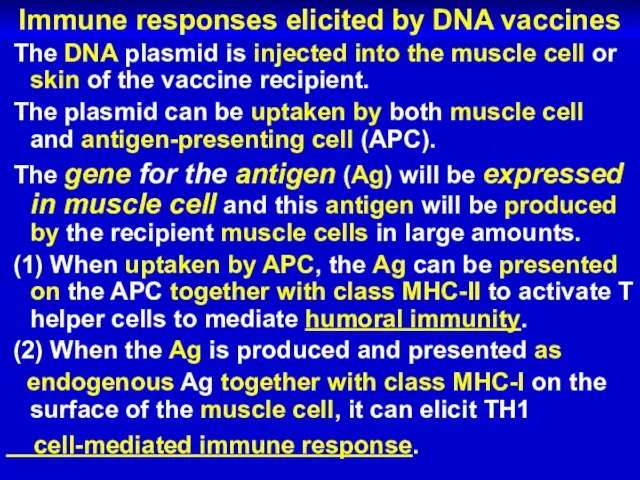
- 69. Immune responses elicited by DNA vaccines The DNA plasmid is injected into the muscle cell or
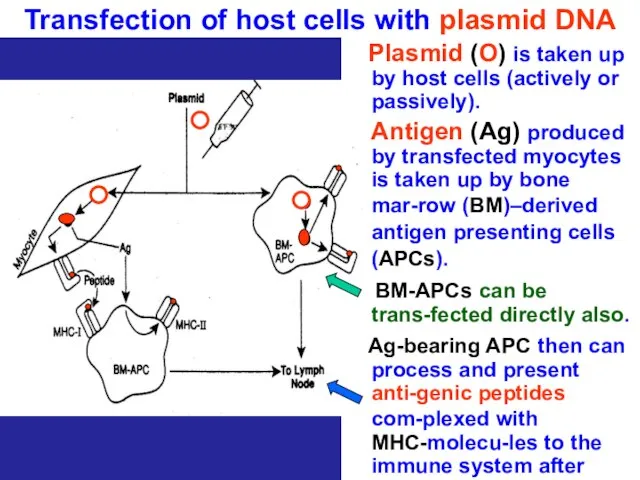
- 70. Transfection of host cells with plasmid DNA Plasmid (O) is taken up by host cells (actively
- 71. DNA Vaccines At present, several different DNA- based vaccines are on clinical trails against malaria, HIV,
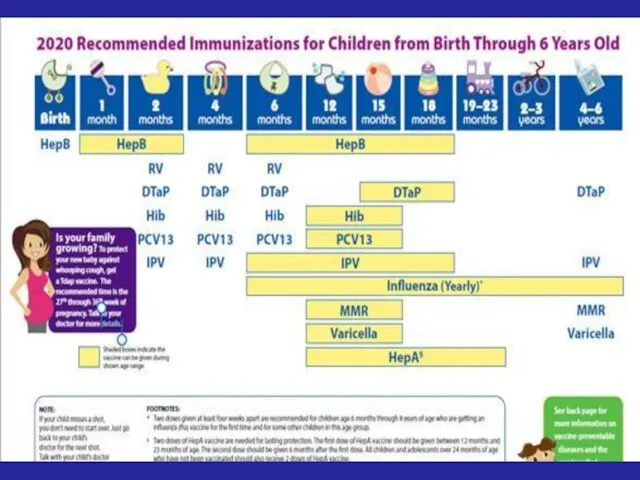
- 72. Recommended Immunization Schedule for
- 76. Скачать презентацию























 Здоровые зубы - гарантия здоровья
Здоровые зубы - гарантия здоровья Как пережить инфаркт, когда вы один
Как пережить инфаркт, когда вы один Заболевания пищеварения
Заболевания пищеварения Вирусы – возбудители респираторных инфекций. Вирус гриппа
Вирусы – возбудители респираторных инфекций. Вирус гриппа Бутират. Оксибутират натрия
Бутират. Оксибутират натрия Тенденция развития ВИЧ-инфекции в России и Брянской области
Тенденция развития ВИЧ-инфекции в России и Брянской области Методы лечения кариеса зубов
Методы лечения кариеса зубов Медико-генетическое консультирование
Медико-генетическое консультирование Мектеп жасындағы балалардың эндокриндік бездері функциясының бұзылуы және олардың алдын алу
Мектеп жасындағы балалардың эндокриндік бездері функциясының бұзылуы және олардың алдын алу Аутизм, РАС-биокоррекция при аутизме и РАС. Интенсив для родителей. Занятие 1
Аутизм, РАС-биокоррекция при аутизме и РАС. Интенсив для родителей. Занятие 1 Гиастат
Гиастат Доказательная медицина в дерматовенерологии
Доказательная медицина в дерматовенерологии Акушерское отделение патологии беременности г. Северодвинск
Акушерское отделение патологии беременности г. Северодвинск Пациент с мигрирующей полиартралгией
Пациент с мигрирующей полиартралгией ГБУЗ КО Кемеровский клинический детский психоневрологический санаторий Искорка
ГБУЗ КО Кемеровский клинический детский психоневрологический санаторий Искорка Дифтерийная инфекция
Дифтерийная инфекция Первая помощь при ДТП
Первая помощь при ДТП Влияние вредных химических факторов на здоровье
Влияние вредных химических факторов на здоровье Үймереттердің ішкі канализация жүйес.і Ішкі канализация жүйесінің негізгі элементтері
Үймереттердің ішкі канализация жүйес.і Ішкі канализация жүйесінің негізгі элементтері Что надо знать об артериальной гипертонии
Что надо знать об артериальной гипертонии Жарақаттың түрлері
Жарақаттың түрлері Безопасное питание
Безопасное питание Иммунопрофилактика. Календарь прививок
Иммунопрофилактика. Календарь прививок Фитотерапия при проявлениях ковидной инфекции в лор-органах
Фитотерапия при проявлениях ковидной инфекции в лор-органах ГИГИЕНА ПИТАНИЯ В МУНИЦИПАЛЬНОМ КАЗЕННОМ ДОШКОЛЬНОМ ОБРАЗОВАТЕЛЬНОМ УЧРЕЖДЕНИИ
ГИГИЕНА ПИТАНИЯ В МУНИЦИПАЛЬНОМ КАЗЕННОМ ДОШКОЛЬНОМ ОБРАЗОВАТЕЛЬНОМ УЧРЕЖДЕНИИ COVID 19 (Коронавирус). Диагностика, возможные варианты лечения
COVID 19 (Коронавирус). Диагностика, возможные варианты лечения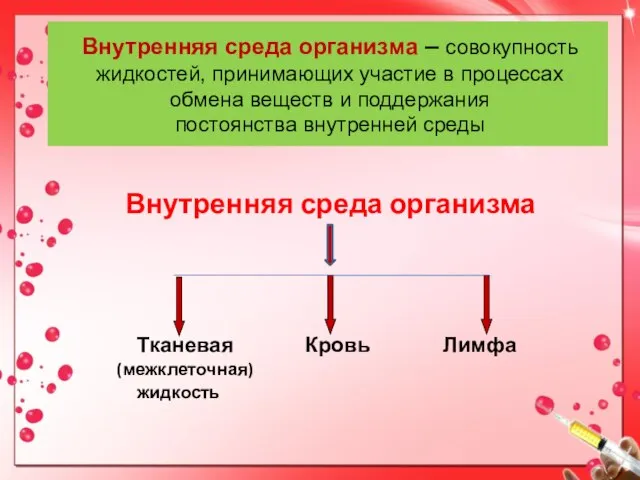 Внутренняя среда организма, клетки крови, кровообращение
Внутренняя среда организма, клетки крови, кровообращение Абдоминальная хирургия
Абдоминальная хирургия