Содержание
- 2. YELLOW FEVER VIRUS Arbovirus Family – Flaviviridae Genus – Flavivirus Single serotype Reservoir - Monkeys Vector
- 3. PAST 1912 – opening of Panama canal – increased global exposure – first modern attempt for
- 4. Hideyo Noguchi, a Japanese bacteriologist – worked for Rockefeller Foundation, Ecuador – Vaccine based on disease
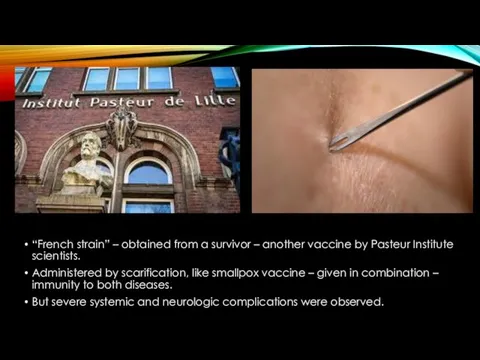
- 5. “French strain” – obtained from a survivor – another vaccine by Pasteur Institute scientists. Administered by
- 6. Attempts to attenuate – failed. Another vaccine developed – derived from Asibi in 1927. First isolation
- 7. In 1937, Max Theiler (awarded the Nobel Prize in Physiology or Medicine in 1951 for developing
- 8. Theiler used chicken eggs to culture the virus. Over 1 million people vaccinated by 1939 –
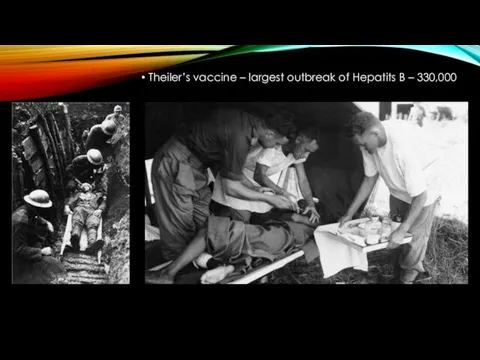
- 9. Theiler’s vaccine – largest outbreak of Hepatits B – 330,000
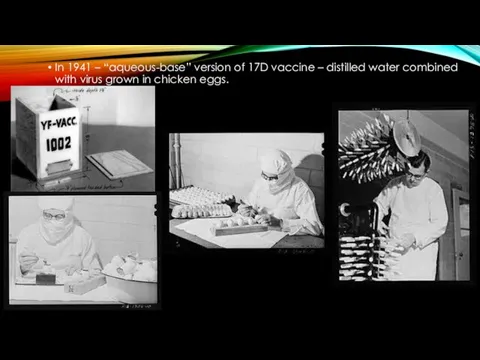
- 10. In 1941 – “aqueous-base” version of 17D vaccine – distilled water combined with virus grown in
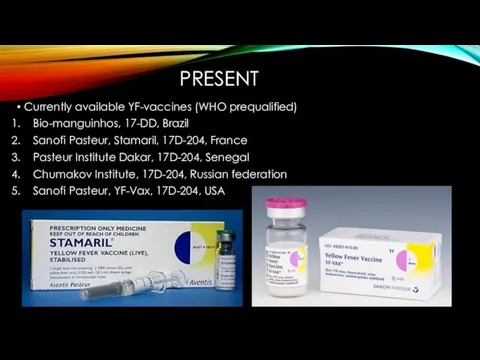
- 12. PRESENT Currently available YF-vaccines (WHO prequalified) Bio-manguinhos, 17-DD, Brazil Sanofi Pasteur, Stamaril, 17D-204, France Pasteur Institute
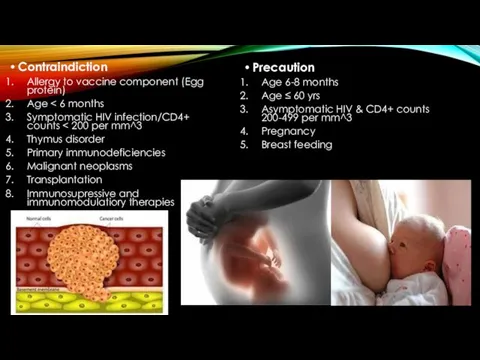
- 13. Contraindiction Allergy to vaccine component (Egg protein) Age Symptomatic HIV infection/CD4+ counts Thymus disorder Primary immunodeficiencies
- 14. Common adverse events of YF Vaccines Fever, headache, backache 3-7 days after vaccination: 5-15% Injection site
- 15. WHO YF vaccines recommendations: SAGE formed YF Vaccine workgroup in 2011: Need for booster dose every
- 16. YF VACCINE ASSOCIATED DISEASE Neurogenic- due to direct viral invasion of CNS or auto-immune mediated, can
- 17. WHO EYE INITIATIVE “Eliminate Yellow Fever Epidemics” Aims to increase 17D vaccine manufacturing to distribute 1.3
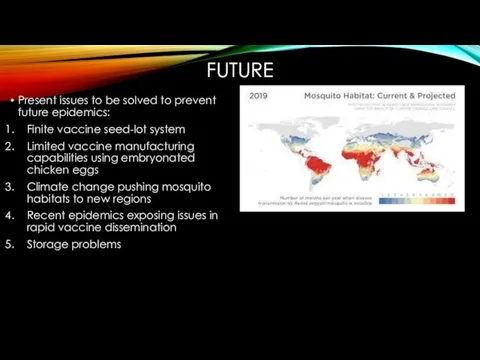
- 18. FUTURE Present issues to be solved to prevent future epidemics: Finite vaccine seed-lot system Limited vaccine
- 19. Solutions A more shelf-stable vaccine - more doses generated with fewer IU per dose YF-Vaccines in
- 21. Скачать презентацию











 Сравнительная характеристика парадонтальных способов обезболивания
Сравнительная характеристика парадонтальных способов обезболивания Кифосколиотические деформации
Кифосколиотические деформации Эпилепсия. Психические расстройства, обусловленные повреждением головного мозга. Умственная отсталость
Эпилепсия. Психические расстройства, обусловленные повреждением головного мозга. Умственная отсталость Тающая с восходом солнца, или влияние манной каши на организм человека
Тающая с восходом солнца, или влияние манной каши на организм человека Поражение молнией
Поражение молнией Особенности внимания младших школьников с детским церебральным параличом
Особенности внимания младших школьников с детским церебральным параличом Характеристика уровней организации медицинской помощи населению в Украине
Характеристика уровней организации медицинской помощи населению в Украине Жертвы космоса
Жертвы космоса Terapeitiskā terapija, kas atbilst vairāk pacientu grupu
Terapeitiskā terapija, kas atbilst vairāk pacientu grupu Профилактика абортов в контексте решения демографической проблемы России
Профилактика абортов в контексте решения демографической проблемы России Физиологические изменения в сердечно-сосудистой и дыхательной системах при беременности
Физиологические изменения в сердечно-сосудистой и дыхательной системах при беременности Значение натрий-калиевого насоса в правильном питании и здоровье человека
Значение натрий-калиевого насоса в правильном питании и здоровье человека Тератомалар
Тератомалар Опорно-двигательный аппарат. Активная часть
Опорно-двигательный аппарат. Активная часть Веб-приложение для учреждения здравоохранения Минская областнаяклиническая больница
Веб-приложение для учреждения здравоохранения Минская областнаяклиническая больница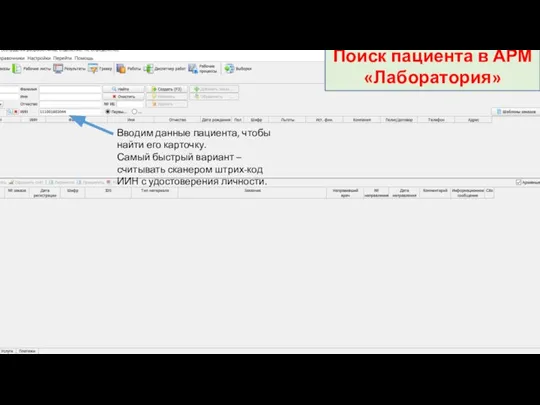 Инструкция по регистрации заказов. Поиск пациента в АРМ Лаборатория
Инструкция по регистрации заказов. Поиск пациента в АРМ Лаборатория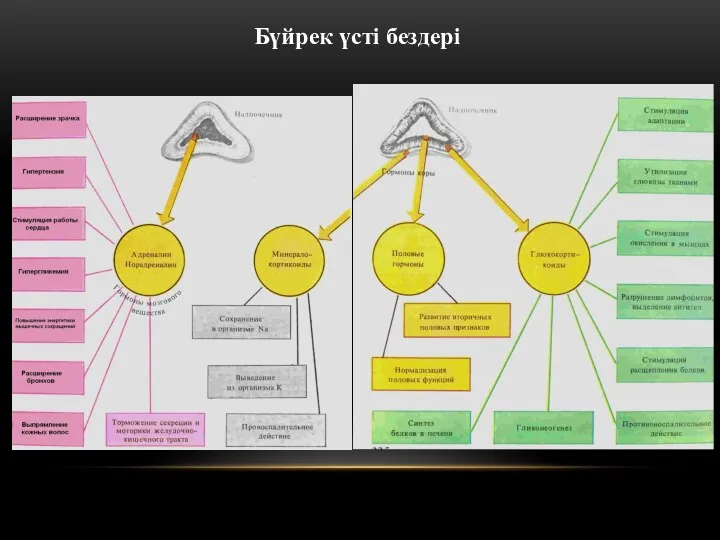 Бүйрук үсті буздері
Бүйрук үсті буздері Рак легкого
Рак легкого Учение о воспалении
Учение о воспалении Кровотечение. Классификация по локализации излившейся крови, по характеру поврежденных сосудов, по скорости истечения
Кровотечение. Классификация по локализации излившейся крови, по характеру поврежденных сосудов, по скорости истечения Оценка факторов, влияющих на течение раннего постэкстубационного периода у больных с острым повреждением легких
Оценка факторов, влияющих на течение раннего постэкстубационного периода у больных с острым повреждением легких Синдром Каннера
Синдром Каннера Система хлебных единиц в питании больных диабетом
Система хлебных единиц в питании больных диабетом Визит в аптеку
Визит в аптеку Осанка и какой она должна быть
Осанка и какой она должна быть Технология Tesla HyperLight® – новая эра в оптике
Технология Tesla HyperLight® – новая эра в оптике Атипті остеомиелит
Атипті остеомиелит