Содержание
- 2. Electrons are arranged in electrons shells (energy levels). The shells have sub-shells (sub-levels). Each shell/sub-shell is
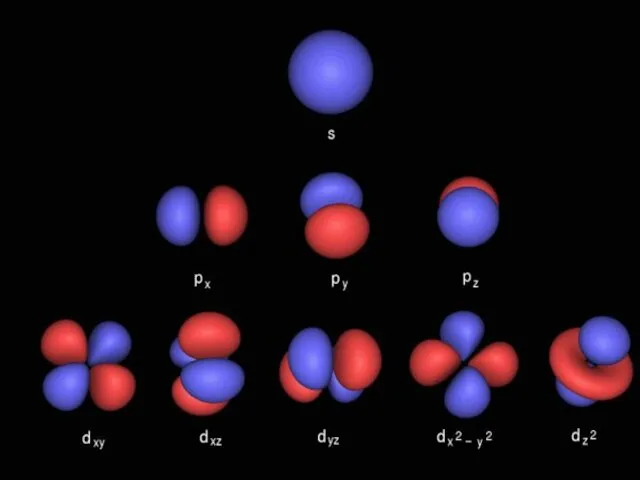
- 3. Each sub-level consists of electron orbitals (region of space in which the electron spends most of
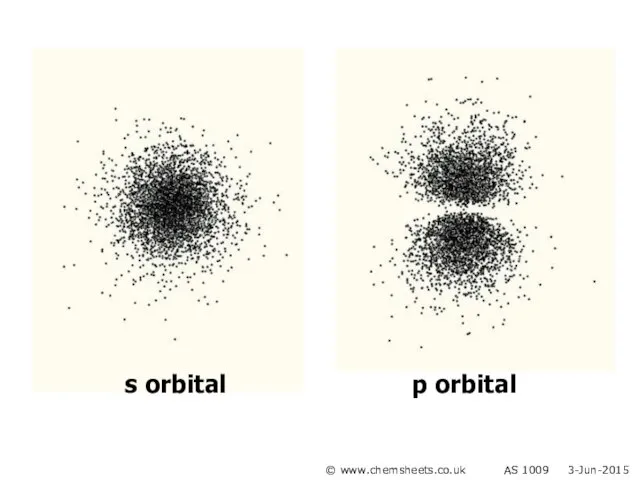
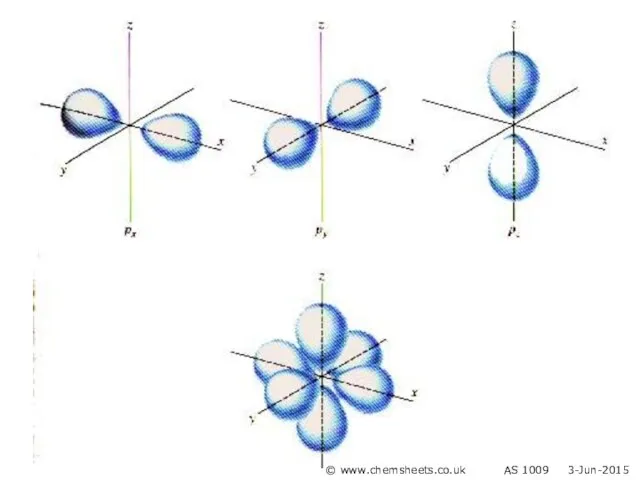
- 4. s orbital p orbital © www.chemsheets.co.uk AS 1009 3-Jun-2015
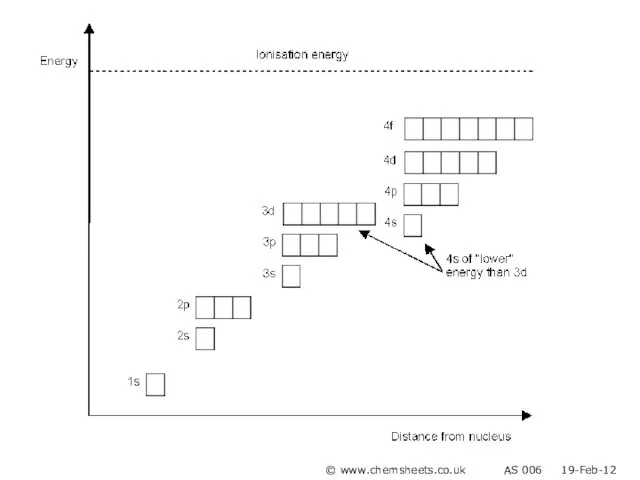
- 5. © www.chemsheets.co.uk AS 006 19-Feb-12
- 6. © www.chemsheets.co.uk AS 1009 3-Jun-2015
- 7. The Orbitron http://winter.group.shef.ac.uk/orbitron/AOs/1s/index.html © www.chemsheets.co.uk AS 006 19-Feb-12
- 8. © www.chemsheets.co.uk AS 006 19-Feb-12
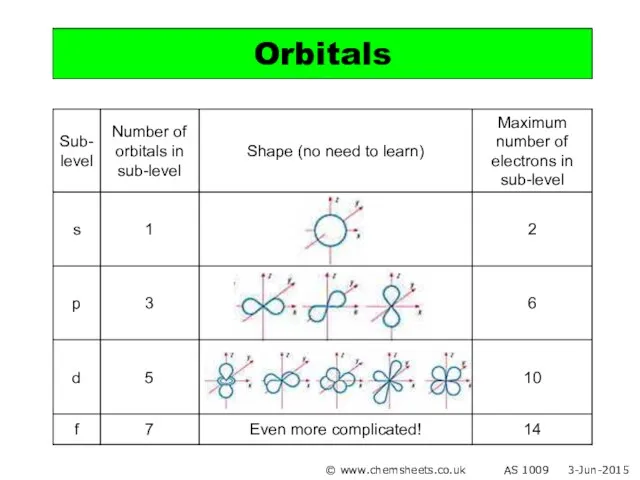
- 9. Orbitals © www.chemsheets.co.uk AS 1009 3-Jun-2015
- 10. other T-shirts are available!!
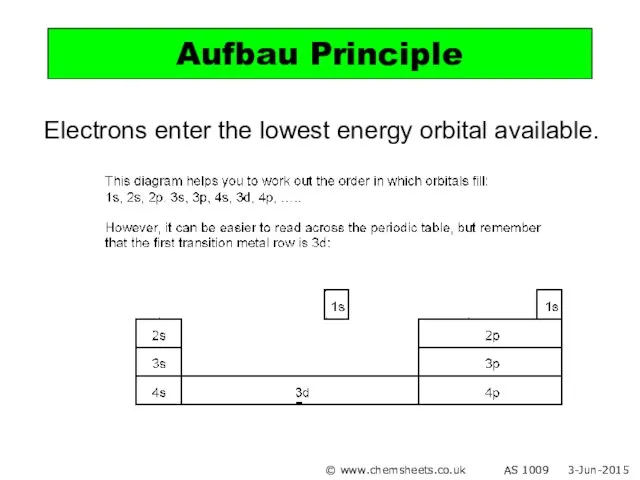
- 11. Electrons enter the lowest energy orbital available. Aufbau Principle © www.chemsheets.co.uk AS 1009 3-Jun-2015
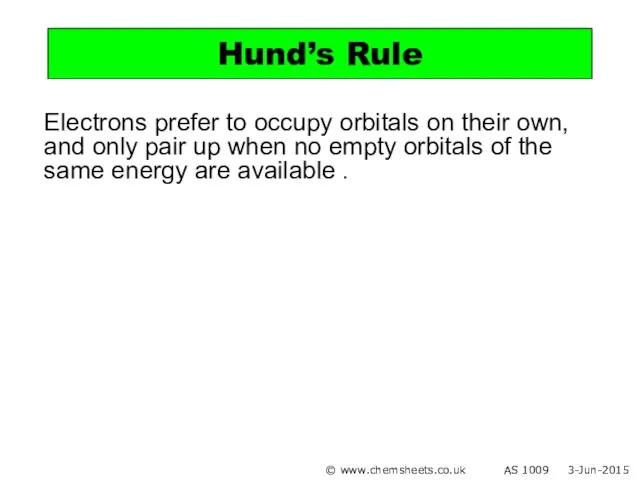
- 12. Electrons prefer to occupy orbitals on their own, and only pair up when no empty orbitals
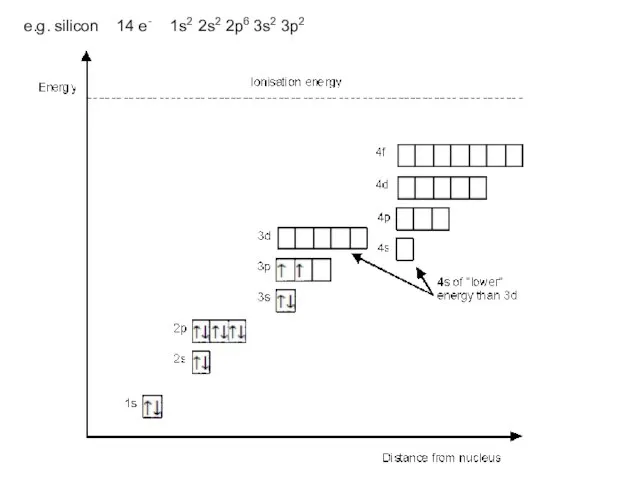
- 13. e.g. silicon 14 e- 1s2 2s2 2p6 3s2 3p2
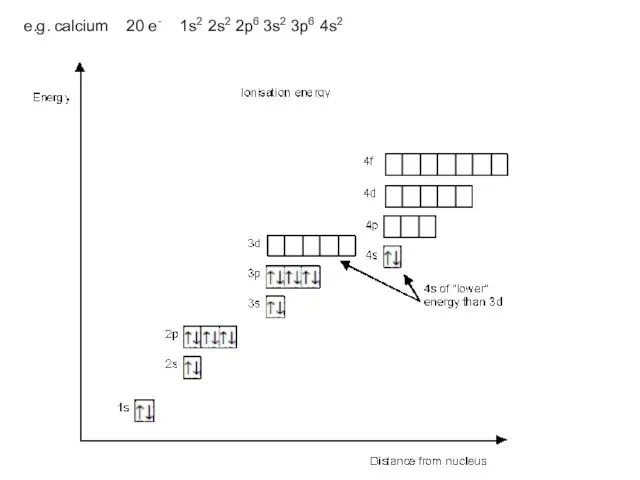
- 14. e.g. calcium 20 e- 1s2 2s2 2p6 3s2 3p6 4s2
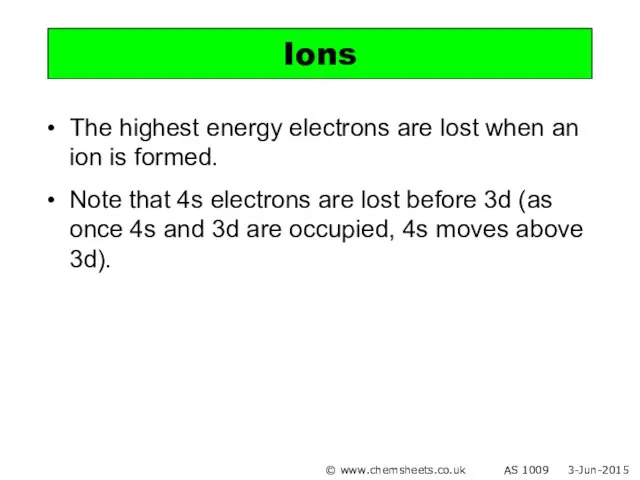
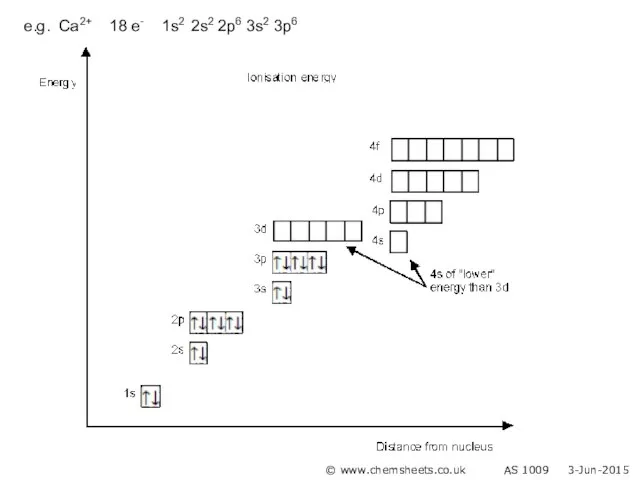
- 15. The highest energy electrons are lost when an ion is formed. Note that 4s electrons are
- 16. e.g. Ca2+ 18 e- 1s2 2s2 2p6 3s2 3p6 © www.chemsheets.co.uk AS 1009 3-Jun-2015
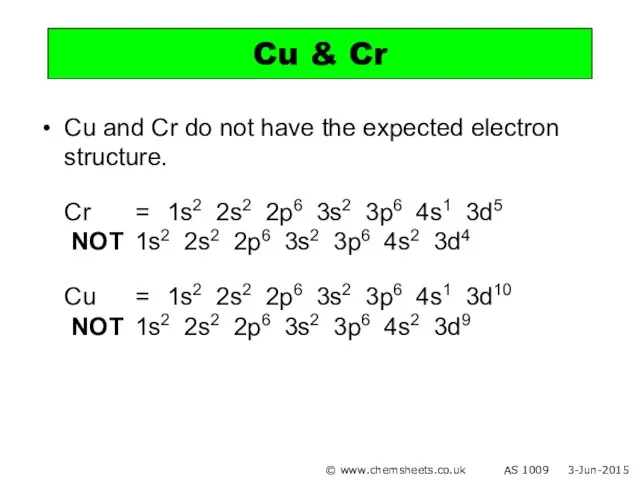
- 17. Cu and Cr do not have the expected electron structure. Cr = 1s2 2s2 2p6 3s2
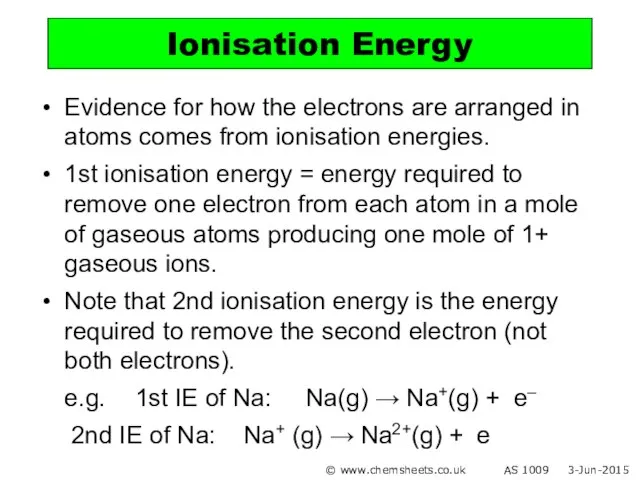
- 18. Evidence for how the electrons are arranged in atoms comes from ionisation energies. 1st ionisation energy
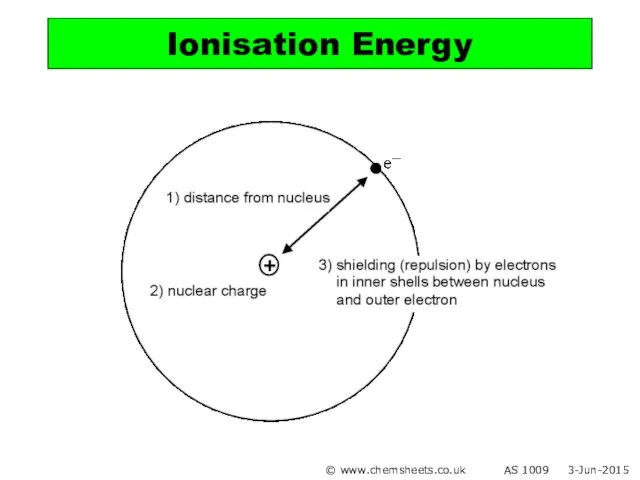
- 19. Ionisation Energy © www.chemsheets.co.uk AS 1009 3-Jun-2015
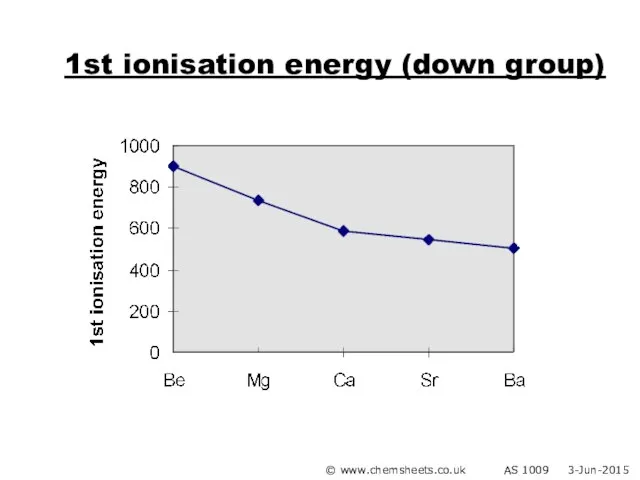
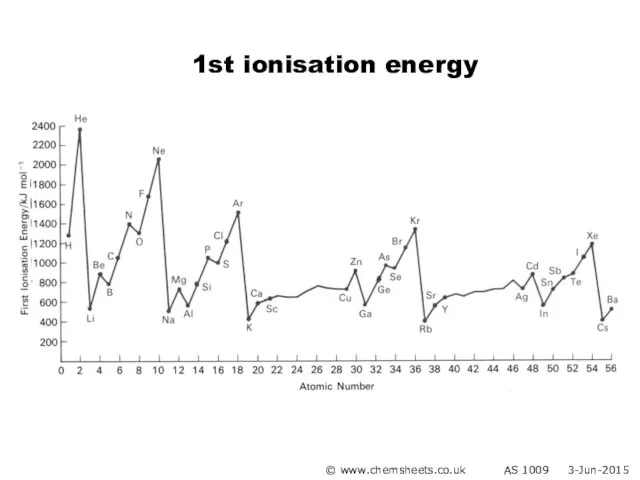
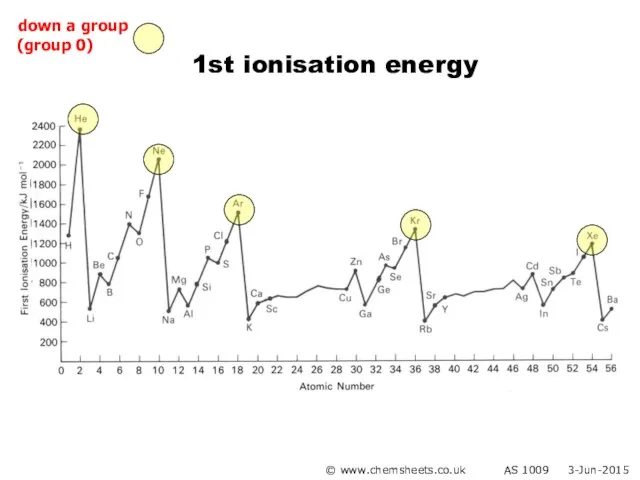
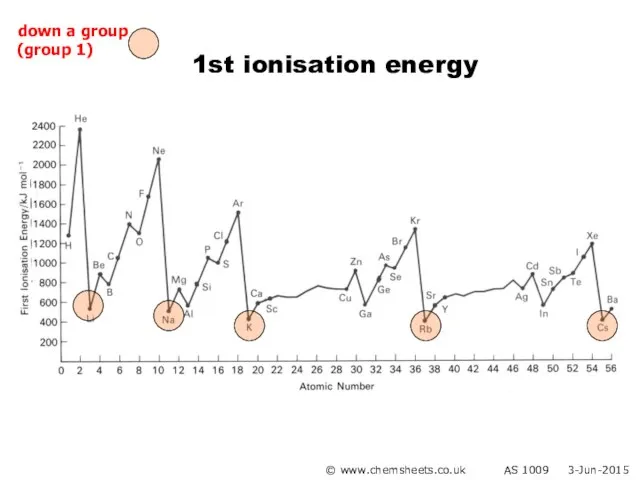
- 20. 1st ionisation energy (down group) © www.chemsheets.co.uk AS 1009 3-Jun-2015
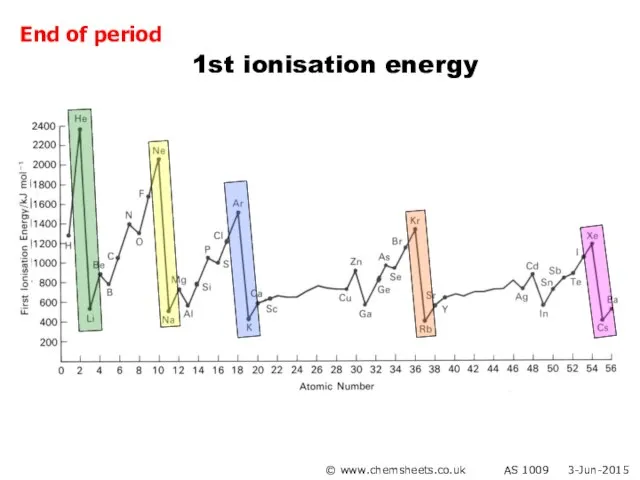
- 21. 1st ionisation energy (down group) Atoms get bigger More shielding Therefore weaker attraction from nucleus to
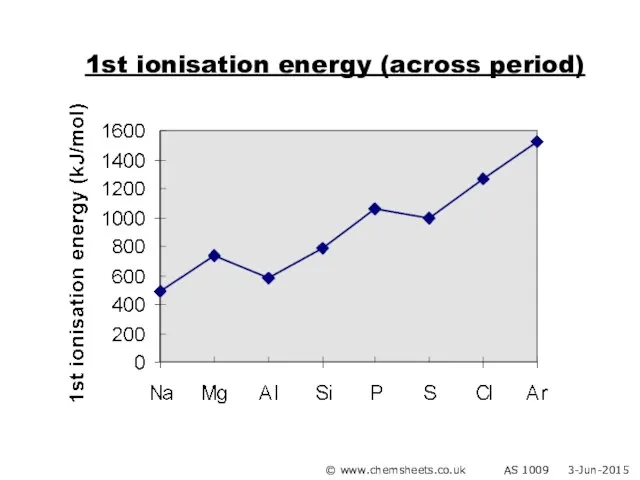
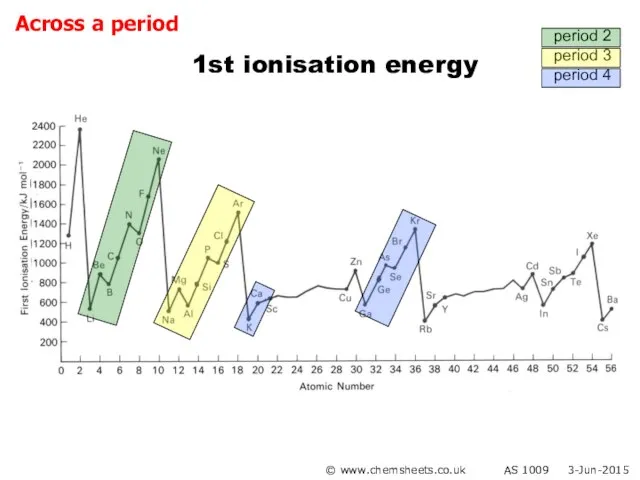
- 22. 1st ionisation energy (across period) © www.chemsheets.co.uk AS 1009 3-Jun-2015
- 23. 1st ionisation energy (across period) General trend Increased nuclear charge (i.e. more protons) Atoms get smaller
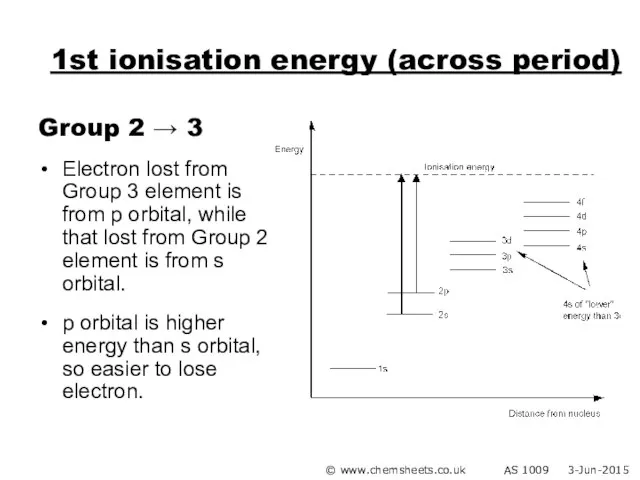
- 24. 1st ionisation energy (across period) Group 2 → 3 Electron lost from Group 3 element is
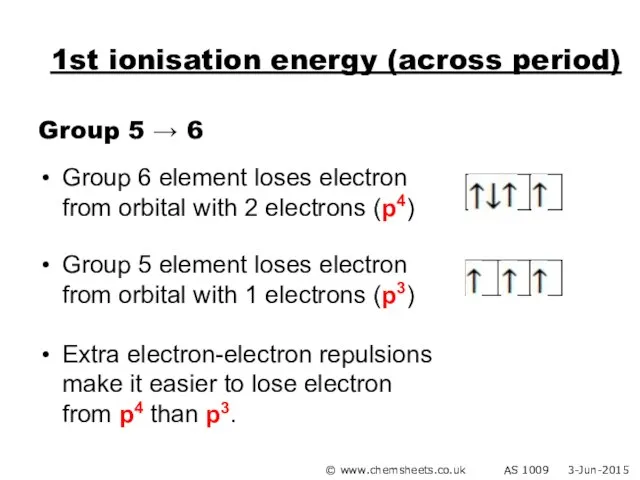
- 25. 1st ionisation energy (across period) Group 5 → 6 Group 6 element loses electron from orbital
- 26. 1st ionisation energy © www.chemsheets.co.uk AS 1009 3-Jun-2015
- 27. 1st ionisation energy © www.chemsheets.co.uk AS 1009 3-Jun-2015 down a group (group 0)
- 28. 1st ionisation energy © www.chemsheets.co.uk AS 1009 3-Jun-2015 down a group (group 1)
- 29. 1st ionisation energy © www.chemsheets.co.uk AS 1009 3-Jun-2015 period 2 period 3 period 4 Across a
- 30. 1st ionisation energy © www.chemsheets.co.uk AS 1009 3-Jun-2015 End of period
- 32. Скачать презентацию




 Электрический ток в металлах
Электрический ток в металлах Электролитическая диссоциация. Устойчивость комплексных соединений
Электролитическая диссоциация. Устойчивость комплексных соединений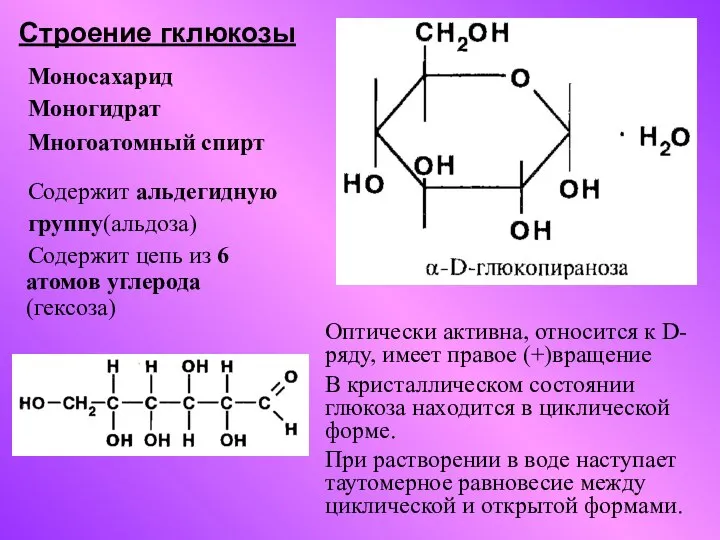 Строение гклюкозы
Строение гклюкозы Ювелирные камни
Ювелирные камни Кислотность почв с.Берёзовка МОУ «СОШ с.Берёзовка» Колесников Иван, 9 класс Пряхина Виктория, 8 класс
Кислотность почв с.Берёзовка МОУ «СОШ с.Берёзовка» Колесников Иван, 9 класс Пряхина Виктория, 8 класс ИКТ на уроках химии. Часть 1. Теория и практика применения интерактивной доски
ИКТ на уроках химии. Часть 1. Теория и практика применения интерактивной доски Целестин
Целестин Гидролиз солей
Гидролиз солей 1. Классификация материалов
1. Классификация материалов Сероводород, сульфиды
Сероводород, сульфиды Реакция полимеризации
Реакция полимеризации Электролиз солей
Электролиз солей Определение содержание фосфатов в воде фотометрическим методом
Определение содержание фосфатов в воде фотометрическим методом Коррозия металлов, сплавов
Коррозия металлов, сплавов Календарь юного химика. 2013-2014 учебный год
Календарь юного химика. 2013-2014 учебный год Валентность. Порядок действий при составлении химической формулы
Валентность. Порядок действий при составлении химической формулы Амины. Номенклатура аминов
Амины. Номенклатура аминов Основи прийняття рішень, щодо захисту населення під час різних фаз радіаційної аварії. Критерії для прийняття рішень
Основи прийняття рішень, щодо захисту населення під час різних фаз радіаційної аварії. Критерії для прийняття рішень Строение и химический состав костей, рост костей
Строение и химический состав костей, рост костей Понятие о спиртах
Понятие о спиртах Оксиды. 8 класс
Оксиды. 8 класс Технология производства и свойства химических волокон и тканей из них
Технология производства и свойства химических волокон и тканей из них Периодическая система химических элементов Д. И. Менделеева
Периодическая система химических элементов Д. И. Менделеева Перманганатометрия и комплексонометрия
Перманганатометрия и комплексонометрия Опасность вещества поливинилхлорида
Опасность вещества поливинилхлорида тэд — без анимации(1)
тэд — без анимации(1) Тема_6б_химическая_кинетика
Тема_6б_химическая_кинетика ad8c00e7ea114b35a6fc29426ea31646 (1)
ad8c00e7ea114b35a6fc29426ea31646 (1)