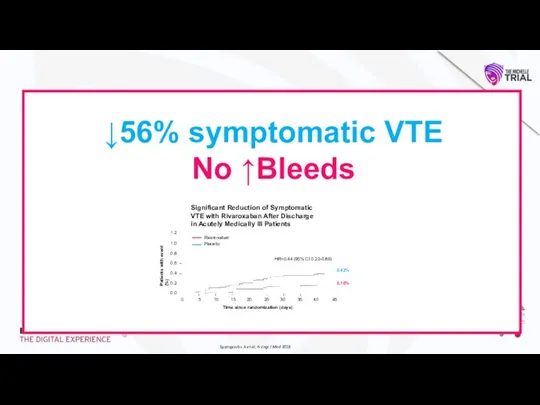
design, conduct or interpretation of the study
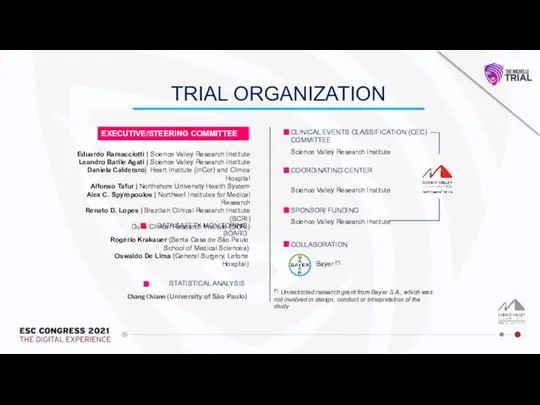
EXECUTIVE/STEERING COMMITTEE
Eduardo Ramacciotti | Science Valley Research Institute
Leandro Barile Agati | Science Valley Research Institute
Daniela Calderaro| Heart Institute (InCor) and Clinics Hospital
Alfonso Tafur | Northshore University Health System
Alex C. Spyropoulos | Northwell Institutes for Medical Research
Renato D. Lopes | Brazilian Clinical Research Institute (BCRI)
Duke Clinical Research Institute (DCRI)
DATA SAFETY MONITORING BOARD
Rogério Krakauer (Santa Casa de São Paulo School of Medical Sciences)
Oswaldo De Lima (General Surgery, Leforte Hospital)
CLINICAL EVENTS CLASSIFICATION (CEC) COMMITTEE
Science Valley Research Institute
COORDINATING CENTER
Science Valley Research Institute
SPONSOR/ FUNDING
Bayer (*)
Science Valley Research Institute
COLLABORATION
Chang Chiann (University of São Paulo)
STATISTICAL ANALYSIS
TRIAL ORGANIZATION






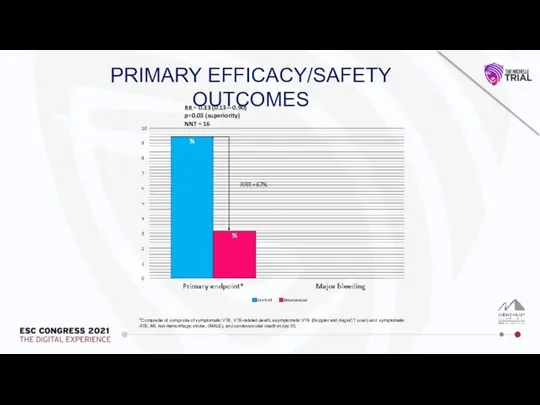
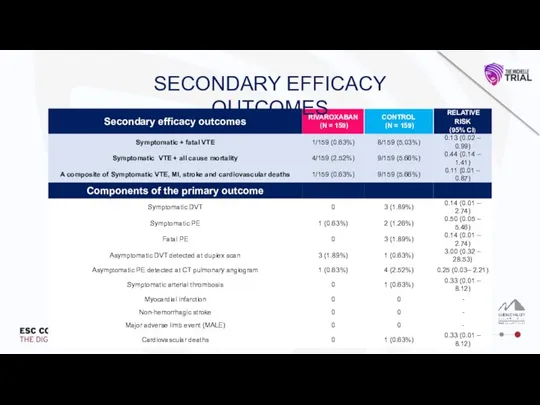
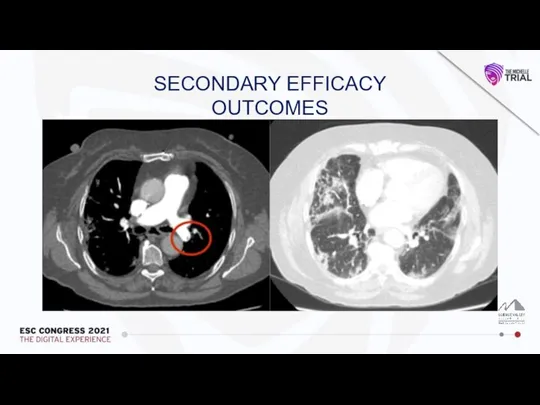
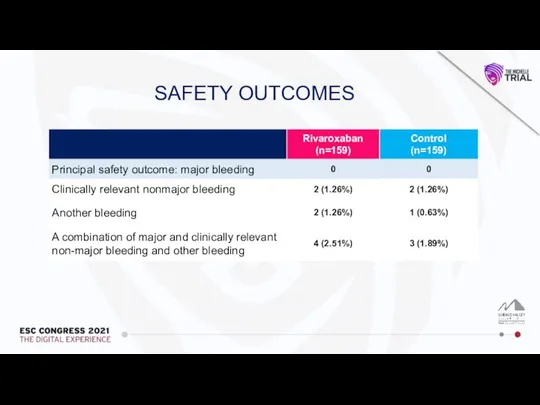

 Влияние алкоголя на печень и ЖКТ
Влияние алкоголя на печень и ЖКТ Органические психические расстройства
Органические психические расстройства Синусит этиология, патогенез
Синусит этиология, патогенез Основные виды нарушений прав ВИЧ-инфицированных
Основные виды нарушений прав ВИЧ-инфицированных Ситуационная задача
Ситуационная задача Disease Escherichia Coli
Disease Escherichia Coli Стандарты выполнения и оценки результатов исследования внешнего дыхания
Стандарты выполнения и оценки результатов исследования внешнего дыхания Dutch eHealth
Dutch eHealth Ведение регистров пациентов с новой коронавирусной инфекцией Covid-19 и внебольничними пневмониями
Ведение регистров пациентов с новой коронавирусной инфекцией Covid-19 и внебольничними пневмониями Пртивоэпидемические мероприятия при дифтерии
Пртивоэпидемические мероприятия при дифтерии Профилактика гнойно-септических заболеваний после родов
Профилактика гнойно-септических заболеваний после родов Доброкачественные и предраковые заболевания шейки матки
Доброкачественные и предраковые заболевания шейки матки Гнойные заболевания кожи и подкожной клетчатки
Гнойные заболевания кожи и подкожной клетчатки Fever during pregnancy, labor and postnatal period
Fever during pregnancy, labor and postnatal period Стандартные подходы к выбору стратегии лечения
Стандартные подходы к выбору стратегии лечения Обеспечение соблюдения требований по обращению с медикаментами,
Обеспечение соблюдения требований по обращению с медикаментами, Паразиты человека
Паразиты человека Влияние прослушивания музыки в наушниках на слух человека
Влияние прослушивания музыки в наушниках на слух человека Биомеханика внешнего дыхания и его протезирования
Биомеханика внешнего дыхания и его протезирования Хроническое легочное сердце
Хроническое легочное сердце Прионные инфекции
Прионные инфекции Профилактика туберкулеза. Лекция №11
Профилактика туберкулеза. Лекция №11 Ожирение
Ожирение Заболевания слёзных органов. Хирургическое лечение
Заболевания слёзных органов. Хирургическое лечение Диагностическая и хирургическая лапароскопия
Диагностическая и хирургическая лапароскопия Ожоги. Ожоги первой и второй степени
Ожоги. Ожоги первой и второй степени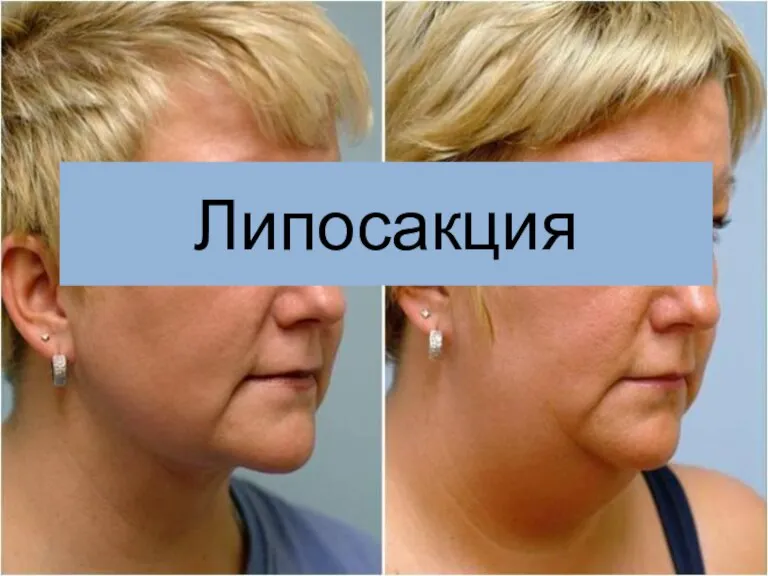 Липосакция
Липосакция Причины рецидива небного абсцесса при патологии верхних моляров и премоляров
Причины рецидива небного абсцесса при патологии верхних моляров и премоляров